Published online Jun 18, 2016. doi: 10.4254/wjh.v8.i17.731
Peer-review started: January 31, 2016
First decision: March 31, 2016
Revised: May 2, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: June 18, 2016
AIM: To assess how the application of different types of markers affects the tracking accuracy of CyberKnife’s.
METHODS: Fifteen patients were recruited and subjected to the ultrasound-guided placement of markers. Two different type of needles 25 gauge (G) and 17 G containing two different fiducial marker, gold notched flexible anchor wire 0.28 mm × 10 mm (25 G needle) and gold cylindrical grain 1 mm × 4 mm (17 G), were used. Seven days after the procedure, a CyberKnife planning computed tomography (CT) for the simulation of radiation treatment was performed on all patients. A binary CT score was assigned to the fiducial markers visualization. Also, the CT number was calculated for each fiducial and the values compared with a specific threshold.
RESULTS: For each patient from 1 to 5, intra-hepatic markers were placed (one in 2 patients, three in 8 patients, four in 3 patients, and five in 2 patients). A total of 48 needles were used (thirty-two 17 G and sixteen 25 G) and 48 gold markers were placed (32 Grain shaped markers and 16 Gold Anchor). The result showed that the CT visualization of the grain markers was better than the anchor markers (P = 5 × 10-9). Furthermore, the grain markers were shown to present minor late complications (P = 3 × 10-6), and the best CT threshold number (P = 0.0005).
CONCLUSION: The study revealed that the Gold Anchor fiducial marker is correlated with a greater number of late minor complications and low visualization by the CT.
Core tip: Robotic radiosurgery can employ different systems for the localization of the neoplastic targets to treat. The purpose of this study is to assess how the application of different types of markers affects the tracking accuracy of CyberKnife’s. Fifteen patients have been recruited and analyzed for the study and two types of markers were used for the procedure. The computed tomography (CT) visualization of grain markers was better than anchor markers P = 5 × 10-9. Grain markers presented minor late complications of P = 3 × 10-6, and the best CT threshold number. The study revealed that the Gold Anchor fiducial marker is correlated with a greater number of late minor complication.
- Citation: Marsico M, Gabbani T, Livi L, Biagini MR, Galli A. Therapeutic usability of two different fiducial gold markers for robotic stereotactic radiosurgery of liver malignancies: A pilot study. World J Hepatol 2016; 8(17): 731-738
- URL: https://www.wjgnet.com/1948-5182/full/v8/i17/731.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i17.731
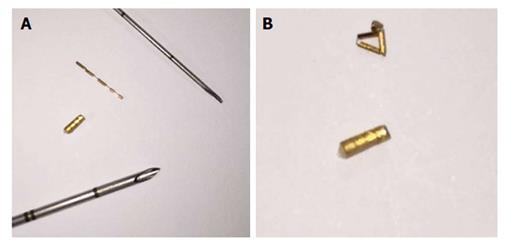
The stereotactic robotic radiosurgery is able to administer high-dose radiation that could reach any anatomic point with a sub-millimeter precision[1-4]. The high accuracy is achieved by the image-guidance system robotic technology and the dynamic tracking of targets, that remove the effect of breathing. The use of these techniques permits the CyberKnife system’s to hit the lesion with high-dose radiation and to safeguard the surrounding critical organs which could suffer irreversible damage[5-13]. Robotic radiosurgery can employ different systems for the localization of the neoplastic targets to treat. In particular, for the treatment of the parenchymatous organ tumors, CyberKnife uses a localization system based on specific gold markers[14]. Various types of gold markers can be employed in relation to the characteristics of the lesion and the different technique of placement. In particular, the type of gold markers to use often depends on the choice of needles of different calibers and length. The choice of the needle is influenced by the type and site of the lesion to treat and its proximity to critical organs or vascular structures[15,16]. The physical characteristic (dimensions and length) of the gold markers strongly depends on the characteristics of the needle. The gold markers (Gold Anchor) contained in fine needle [25 gauge (G) and 22 G] must be smaller in dimension and longer than those contained in larger needles. Markers contained in fine needles, in order to reach an appropriate density for a normal computed tomography (CT) number and to be correctly recognized by the CyberKnife system, must assume a correct array in the parenchyma, when they are inserted. In fact, they have the advantage of being flexible and to curl up when they are pushed against the parenchyma tanks to the spindle and carried by the needle. Therefore, after their placement, the Gold Anchor reached some similar dimensions to those in grain and so, an appropriate density and a normal CT number. Therefore, if they are too crowded or shatter during their release, they do not achieve the proper density to have a normal CT number, and to be well recognized as a fiducial by the CyberKnife System. The markers (cylindrical markers) contained in larger gauge needles (17 G and 18 G) can not break and do not need to mass during their placement. Therefore they can not change their CT number (Figure 1)[17-21]. The placement of fiducial markers may be burdened by complications due to puncture or related to the gold markers. For instance, the major complications related to the gold markers could be the migration of fiducials from the positioning site and the physical alterations of the markers, like marker not deployed or shattered, that may occur during or after placement[22-26]. These complications determine the lack of fiducials recognition by CyberKnife and result to failure in targeting the lesions that prevented the execution of the treatment[27,28].
The aim of this prospective pilot study was to assess, how the use of two different types of gold fiducial markers: Grain type and Anchor type, affects the accuracy of tracking by the CyberKnife System, and consequently, the therapeutic efficacy of the treatment of primary or metastatic liver malignancy. We also aimed to identify which type of fiducial can ensure better viability of the SRR.
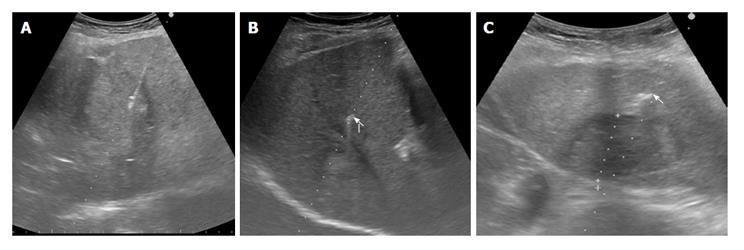
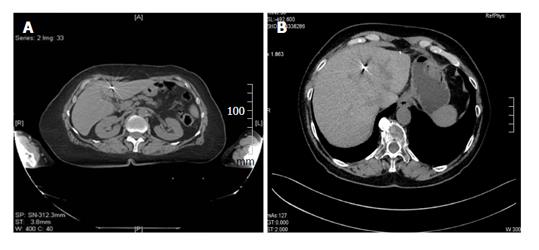
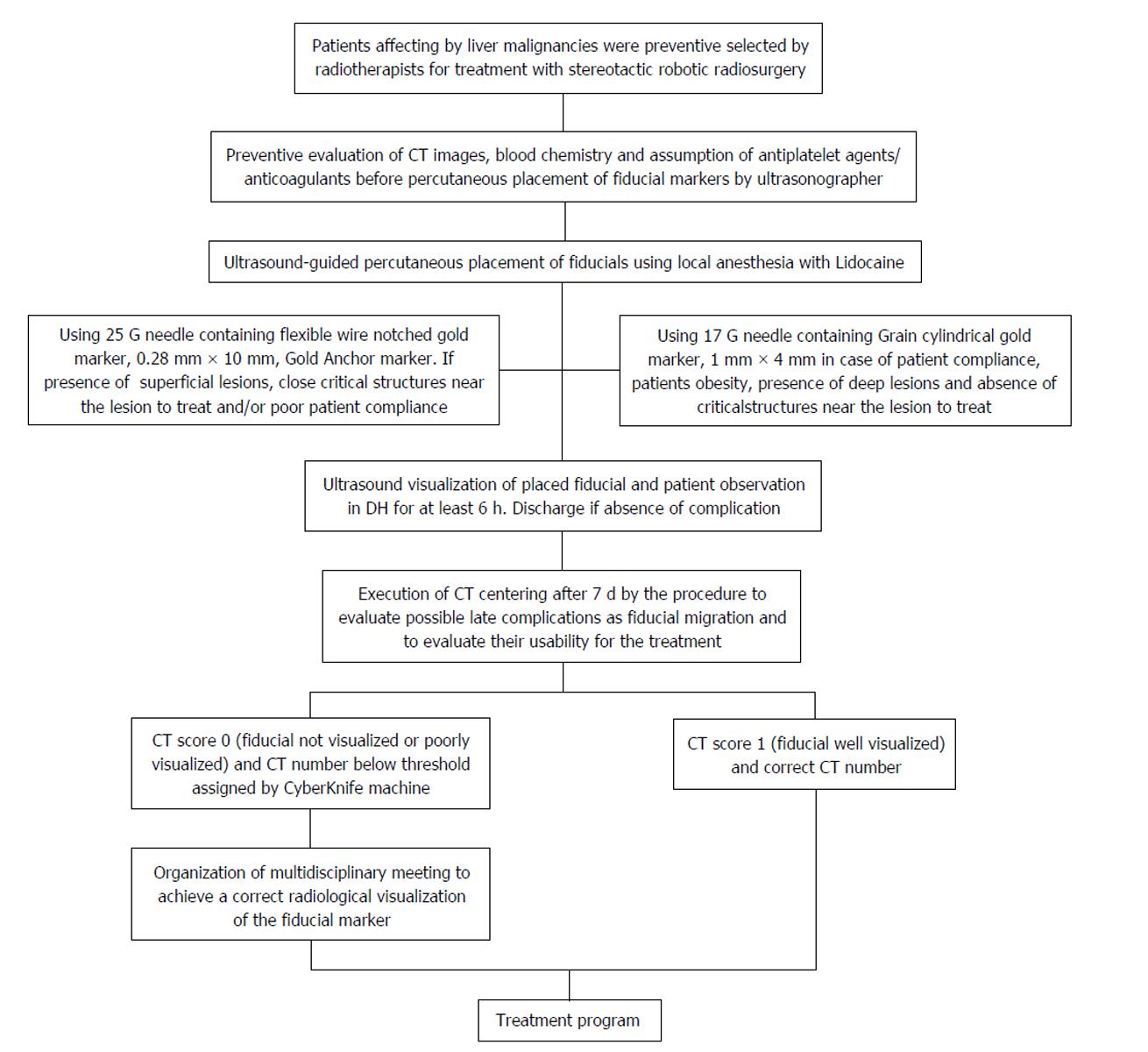
Fifteen consecutive patients, who were scheduled to receive robotic radiotherapy treatment for primary or metastatic liver malignancy, were recruited for percutaneous ultrasonography (US)-guided placement of intra-hepatic fiducial markers, from March 2014 to June 2014 (Figure 1). A written informed consent was obtained from the patients. Two different types of needles, 25 G and 17 G containing two different fiducial markers, gold notched flexible anchor wire of 0.28 mm × 10 mm (25 G needle) and gold cylindrical grain of 1 mm × 4 mm (17 G), were used. The needle type to use was selected according to the site of the lesion (deep or superficial liver lesion) and physical structure. The choice of the different fiducial markers depends mainly on the choice of the needle caliber. The number of fiducial markers to place was evaluated according to the acoustic window, the compliance of the patients and morphological characteristics of the lesions. The examination was performed by two expert ultrasonographers with the same echograph, ProSound Alfa7, (Hitachi-Aloka, Tokyo, Japan) with a 3.75-7.5 MHz hemispheric sound technology (HST) 91-30 Multi Frequency Convex Abdominal HST probe. Local anesthesia was achieved via the subcutaneous administration of 1% lidocaine. All the gold fiducial markers were placed with US-guidance through sub or intercostals access. After confirming that the needle tip had reached the target lesion, the fiducial marker was deployed, and then the needle was removed. We placed in each patient from 1 to 5 fiducial markers, and when at least two or more fiducials were placed, it was at a distance of about 1.5-2 cm apart, in a way to occupy the perpendicular edges of a cube containing the tumor inside. The Gold Anchor markers were always placed with the same technique to take advantage of their mass effect. Fiducial positioning was confirmed with ultrasound image. A marker was usually seen as a hyperechoic structure. The two different fiducial markers used were sonographycally undistinguishable (Figure 2). Technical success was defined when the implantation enables adequate treatment planning and CT simulation. Fiducial migration was defined as seed dislodgement outside the volume of the original injection site that is unusable for guiding stereotactic body radiation therapy (SBRT) as determined by planning CT. Clinical success was defined as the completion of SBRT. Seven days after the procedure, a CyberKnife planning CT for the simulation of radiation treatment was performed on all patients. A binary CT score for the fiducial markers visualization was assigned (not visualized or poorly visualized = 0; well visualized = 1) (Figure 3). In the case of CT score of zero (0) which prevented treatment, we organized a series of multidisciplinary meetings (with regards to the procedure, the physician and the radiation oncologist responsible for the radiosurgery treatment) to achieve the correct radiological visualization of the fiducial marker. Moreover, for the execution of treatment with CyberKnife, it is necessary that each fiducial reaches a CT number above a specific threshold (CT number threshold). The CT number of the fiducial is assigned in an automated manner by the CyberKnife machine (Figure 4). Database construction and data analysis were performed using Office Excel 2007, XLSTAT 2016 (microsoft) and SPSS for Windows (SPSS Inc., Chicago, United States). We examined the data with the use of appropriate parametric and non-parametric statistical tests (Student’s t-test two-tailed and a χ2 test according to Fischer considering P < 0.05 as significant). A Lilliefors (Kolmogorov-Smirnov) test for normality has been previously performed. Statistical analysis was performed by Tommaso Gabbani, MD, and reviewed by Principal Investigator, Maria Marsico, MD.
Fifteen consecutive patients (men: 9, women: 6, mean age: 72.9 years old, range: SD ± 7.9) who had already undergone percutaneous ultrasound-guided fiducial marker implantations for CyberKnife therapy were employed for this study. Eleven patients (8 males) presented liver metastasis from a note primary neoplasm (2 right colon carcinoma, 2 sigmoid carcinoma, 2 rectum carcinoma, 1 gastric carcinoma, 1 lung carcinoma, 1 ovaric carcinoma and 2 pancreatic carcinoma). Four patients (2 males) showed liver primary malignancy [2 hepatocellular carcinoma (HCC), 1 cholangio-carcinoma, 1 hepatic cholangio-carcinoma]. Among 11 patients who presented liver metastasis, 9 patients had previously undergone radical surgery of primary neoplasm. Among these 9 patients, 4 had submitted to adjuvant therapy, 2 to metastasectomy and adjuvant therapy, 2 to neo-adjuvant and adjuvant therapy and 1 to metastatectomy without chemotherapy. Moreover among these 9 patients, 7 developed new liver metastasis during or at the end of the treatment, while 2 patients presented a metastatic recurrence. Only 2 patients of the 11 affected by liver metastasis undergone treatment by chemotherapy and not surgery, one performed a palliative chemotherapy and the other performed an effective chemotherapy with failure of treatment. In the group of patients with primary hepatic neoplasm, patients affected by HCC and hepato-cholangiocarcinoma were treated by chemoembolization, while another one affected by cholangiocarcinoma undergone chemotherapy. Patients treated with chemoembolization showed relapse of neoplasm, while the patient treated with chemotherapy showed no response to the treatment. In the group of patients with hepatic metastasis, 8 of them have a single nodule, 2 of them have two nodules, and 1 has three nodules so the total of liver lesions treated was 15. These 15 liver lesions presented a maximum diameter between 2 to 4 cm. In the group of patients with primary liver lesions, 3 patients showed a single nodule and another one presented two nodules so the total primary lesion treated was 5. Four of these measured a maximum diameter from 2 to 4 cm and only one measured a maximum diameter over 4 cm. 2 patients showed a moderate ascites at the moment of the procedure. The 20 liver lesions were localized into the VII liver segment (n = 7), VI liver segment (n = 3), VIII segment (n = 3), V segment (n = 1), IV segment (n = 1), III segment (n = 1), between V-VI segment (n = 1), between V-VI-VII segments (n = 1) and between VI-VII segments (n = 2). Five lesions were localized close to vascular structures and 2 lesions close to critical organs. Considering the closeness of the critical organs or vascular structures and the patients’ compliance, 8 patients undergone a combined placement of the two types of gold markers. Two patients presented severe compliance problems (panic attack), so they received only anchor markers (placed with fine needles). Five patients received only cylindrical grain markers. For each patient about 1 to 5 intra-hepatic markers were placed (one in 2 patients, three in 8 patients, four in 3 patients, and five in 2 patients). A total of 48 needles were used (thirty-two 17 G and sixteen 25 G) and 48 gold markers were placed (32 Grain shaped markers and 16 Gold Anchors). In 47 cases, the gold markers were placed through subcostal access and only in a single case with an inter-costal access. Every patient received a local anesthesia with lidocaine. All fiducials placement were sonographically confirmed right after the procedure. No patient presented any major complication related to the procedure.
After the placement of markers, 14 patients underwent the planning simulation CT scan to allow fiducials to settle. One patient did not perform the CT because of a complication related to the primary tumor (hepatic failure). Removing the latter patient who was excluded from the treatment for causes not correlated to the fiducial placement, the technical and clinical success rate was 100%. The CT scan revealed that 14 markers (11 Gold Anchors and 3 Grain shaped markers) showed late complications. Few markers showed more than one complications at the same time for a total of 27 complications. Shattered markers (n = 2; 2 Gold Anchors), extra-hepatic migration (n = 4; 1 Gold Anchor and 3 Grain markers), extra-hepatic migration and marker not visualized (n = 1; 1 Gold Anchor), intra-hepatic migration (n = 5; 5 Gold Anchors), not massed markers (n = 5; 5 Gold Anchor). The Gold Anchor marker presented more frequent late minor complications (68.75% vs 9.375%, P = 3 × 10-6). Moreover, 38 markers were visualized with CT score = 1 and 10 markers with CT score = 0, the markers visualized with CT score = 0 were all Gold Anchors and we demonstrated that the CT subjective visualization of Grain shaped markers was significantly higher than the CT subjective visualization for Gold Anchor (100% vs 37.5%, P = 5 × 10-9). For 5 patient it was necessary to organize multidisciplinary meetings to identify the correct intra-hepatic localization of the markers visualized with CT score = 0. Finally, 5 markers showed a CT number below the threshold (5 Gold Anchors). The 5 markers with the CT number below the threshold were not recognized by the CyberKnife system and so were not used for the treatment (one marker not recognized for 5 patients). Forty-three markers (32 Grain shaped markers and 11 Gold Anchors) with regular CT number were recognized by CyberKnife system and were used for the treatment. The clinical success achieved was 89.6%. We demonstrated that the Gold Anchor marker is associated with a threshold below the CT number (31.25% vs 0%, P = 0.0005) that is not suitable for treatment. A total of 14 patients underwent radiosurgery treatment, only one patient was excluded because of a complication related to his primary tumor.
The CyberKnife Robotic Radiosurgery System is a non-surgical option for patients who have inoperable or surgically complex tumors or who may be looking for an alternative to surgery. It is an option in the case where no response and/or relapse is observed after chemotherapy and standard radiotherapy[29-31]. In our study, we compared the therapeutic usability of the two different gold fiducial markers for robotic radiosurgery treatment of primary and metastatic liver malignancies. We used the two different gold markers according to the necessity to either use 17 or 25 G needle, depending on the patient’s compliance, patient physical structure and the proximity of critical or vascular structures. This pilot trial demonstrate that the Anchor marker (0.28 mm × 10 mm) is correlated with a greater number of late minor complications that results from a frequent association with a CT number below threshold and a low subjective CT visualization, resulting in a delay or a difficulty in starting the treatment. In our opinion, the use of the Gold Anchor marker should be limited to use of the 25 G needle and in combination with the other types of markers. Only few studies have compared the use of different fiducial in the terms of efficacy and complications[32,33]. Our study differs from others because it compares the two different types of gold fiducial markers in terms of usability for CyberKnife treatment. In our study, we identified some of the factors related to the type of fiducials (the Gold Anchor) that may prevent the treatment with CyberKnife. The identification and knowledge of these factors allows us to limit the use of Gold Anchor marker type, to specific cases and preferably, in combination with the other marker types, in order to reduce the tracking problems of CyberKnife. Infact, CyberKnife tracking problems are causes of increasing costs and delay in treatment execution. Contrary to what is shown in our study, other trials have demonstrated the advantage of Gold Anchor fiducial than the other types of fiducial markers for the treatment with CyberKnife. Nevertheless, in these other studies, inserting of the fiducial markers was executed by endoscopic ultrasonography technique to treat tumors of the pancreas and lung. Therefore, it is our opinion that the different techniques for positioning, and the different localization of the lesions may be the basis of the different results obtained in the study. Furthermore, we must consider the variable offered by the needle. The percutaneous placement of Gold Anchor (0.28 mm × 10 mm) occurred with the 25 G needle that originally contained the gold marker. In the cases of endoscopic ultrasound guided placement of fiducial gold markers (in particular, for treatment of pancreas lesion), the 25 G needle originally containing the marker, serves only as a carrier to put the fiducials inside the other needles (22 G or 19 G) usually used for the fiducial placement. The use of needles with a greater caliber (22 G and 19 G) and less flexibility than the 25 G needle, may facilitate the placement of Gold Anchor limiting the complications.
The treatment of liver malignancies has evolved over the years. Although surgery is the current standard treatment for localized surgically operable lesions. Alternative treatment approaches for unresectable liver metastasis and primary liver cancer include: Chemoembolization, radiofrequency ablation, cryotherapy, and the oral multikinase inhibitor sorafenib, chemotherapy and standard radiotherapy. The CyberKnife Robotic Radiosurgery System is a non-surgical option for patients who have inoperable or surgically complex tumors or who may be looking for an alternative to surgery. It also provides an option in the case where no response and/or relapse is observed after standard treatment.
Many points still remain unclear in literature to ameliorate the treatment by CyberKnife and a lot of them seem to correlate with the type of fiducial to be use, the technique of placement and the number of fiducial to use. Nowadays, there are many different types of gold fiducial markers with different dimensions, lengths and physical characteristics. Therefore, many other studies of fiducial comparison, like the authors’, should be conducted. This is necessary to identify the basis of compliance, demographic and clinical characteristics of the patients and liver lesions characteristics, the best type of fiducial and needle to use. Percutaneous fiducial marker placement could be under computed tomography (CT) fluoroscopic guidance or ultrasonographic (US) guidance. In this study, fiducial placement was entirely conducted under US guidance demonstrating a great safety and efficacy. Cost-effectiveness studies should also be conducted to compare the CT and the US percutaneous fiducial placement to identify the best method in the terms of cost-effectiveness. Nowadays, there is yet no consensus in literatures on the exact number of fiducials necessary to effectively perform the treatment with CyberKnife. Many studies define the technical success as the ability to place more of a fiducial near the tumor target before the treatment; other studies have resulted in higher clinical success placing a unique fiducial marker for patient. In this study, the authors also demonstrated a high clinical success from using one to five fiducial for each patient, in relation to which fiducials were really recognized and used by the CyberKnife system (fiducial with correct relegated CT number). Therefore, many studies should be conducted regarding the different analysis of tracking accuracy resulting from the use of a different number of fiducial for treatment. This is important to establish the best number of fiducials to use in terms of cost effectiveness.
The study differs from others because it compares the two different types of gold fiducial markers in terms of usability for CyberKnife treatment of liver malignacies. The study also differs for describing other possible complications related to Gold Anchor - their wrong stacking and their break after the placement. In this study, the authors identified some factors related to the type of fiducial, (the Gold Anchor) that may prevent the treatment with CyberKnife. The identification and knowledge of these factors allows them to limit the use of Gold Anchor marker type, to specific cases and preferably in combination with other marker types, in order to reduce tracking problems of CyberKnife. In fact, CyberKnife tracking problems are causes of increasing costs and delay in treatment execution. Contrary to what is shown in the study, other trials have demonstrated the advantage of Gold Anchor fiducial over the other types of fiducial markers for the treatment with CyberKnife. Nevertheless, in these other studies, the inserting of fiducial markers was executed by endoscopic ultrasonography technique for treating tumors of the pancreas and lung. Therefore, it is the authors’ opinion that the different techniques of positioning, and the different localization of the lesions and the different needles (generally of higher caliber) used for the placement of Gold Anchor may be the basis of the different results obtained in this study.
In the authors’ opinion, the use of Gold Anchor marker type has to be limited to specific cases; in order to reduce tracking problems of CyberKnife treatment which is the major cause of increasing costs and delay in treatment execution. Therefore, the authors suggest that the use of the Gold Anchor marker should be limited to the necessity to use the 25 G needle and in combination with the other type of markers. In particular, the 25 G needle should be used in the case of low patient compliance, absence of obesity and in the presence of superficial lesions at critical structure near the liver lesions.
Stereotactic robotic radio surgery: Ability to dispense high doses of focused radiation in a minor number of fractions respect to the standard treatment (2-5 vs 30-40). Ability to reach any point with anatomical precision and extreme sub-millimeter accuracy tanks to a target localization computerized system offered by CyberKnife system; CyberKnife: Robot with a complete autonomy characteristic with more than 1500 dispensing positions of X-ray; Variable diameter collimator; Synchrony Respiratory Tracking System to preserve the near organs from toxicity; Fiducial gold markers: Markers exploited by CyberKnife for the target localization in the treatment of parenchymatous organs lesions. This marker is made from gold, which makes it biocompatible and ensures it exhibits good contrast on X-ray images; CT number: A normalized value of the calculated X-ray absorption coefficient of a pixel (picture element) in a computed tomogram, expressed in Hounsfield units, where the CT number of air is -1000 and that of water is 0.
In this work, the authors reported a comparison study of two different types of fiducial markers for robotic radiosurgery. In this study, 15 patients have been recruited, in which 48 gold markers were placed (32 Grain shaped markers and 16 Gold Anchor). All these patients except one were scanned with CT for visualization and identification of these markers. The data of these patients were analyzed and reported in this work. The work intended to address an interesting clinical issue.
P- Reviewer: Chang Z, Tomuleasa C S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, Grosu AL, Guckenberger M. Stereotactic radiosurgery for treatment of brain metastases. A report of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol. 2014;190:521-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Herfarth KK. Extracranial stereotactic radiation therapy. New Technologies in Radiation Oncology. Berlin: Springer-Verlag 2006; 277-288. [DOI] [Cited in This Article: ] |
| 3. | Seo Y, Kim MS, Yoo S, Cho C, Yang K, Yoo H, Choi C, Lee D, Kim J, Kim MS. Stereotactic body radiation therapy boost in locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2009;75:1456-1461. [PubMed] [Cited in This Article: ] |
| 4. | Kalogeridi MA, Zygogianni A, Kyrgias G, Kouvaris J, Chatziioannou S, Kelekis N, Kouloulias V. Role of radiotherapy in the management of hepatocellular carcinoma: A systematic review. World J Hepatol. 2015;7:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 106] [Article Influence: 11.8] [Reference Citation Analysis (2)] |
| 5. | Collins BT, Vahdat S, Erickson K, Collins SP, Suy S, Yu X, Zhang Y, Subramaniam D, Reichner CA, Sarikaya I. Radical cyberknife radiosurgery with tumor tracking: an effective treatment for inoperable small peripheral stage I non-small cell lung cancer. J Hematol Oncol. 2009;2:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Li D, Kang J, Golas BJ, Yeung VW, Madoff DC. Minimally invasive local therapies for liver cancer. Cancer Biol Med. 2014;11:217-236. [PubMed] [Cited in This Article: ] |
| 7. | Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol. 2013;8:118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Choi BO, Jang HS, Kang KM, Lee SW, Kang YN, Chai GY, Choi IB. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:154-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 414] [Cited by in F6Publishing: 395] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 10. | Matsuo Y, Onishi H, Nakagawa K, Nakamura M, Ariji T, Kumazaki Y, Shimbo M, Tohyama N, Nishio T, Okumura M. Guidelines for respiratory motion management in radiation therapy. J Radiat Res. 2013;54:561-568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Wong JW, Sharpe MB, Jaffray DA, Kini VR, Robertson JM, Stromberg JS, Martinez AA. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44:911-919. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 741] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 12. | Lohr F, Debus J, Frank C, Herfarth K, Pastyr O, Rhein B, Bahner ML, Schlegel W, Wannenmacher M. Noninvasive patient fixation for extracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1999;45:521-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Kubo HD, Hill BC. Respiration gated radiotherapy treatment: a technical study. Phys Med Biol. 1996;41:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 503] [Cited by in F6Publishing: 513] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 14. | Ohta K, Shimohira M, Murai T, Nishimura J, Iwata H, Ogino H, Hashizume T, Shibamoto Y. Percutaneous fiducial marker placement prior to stereotactic body radiotherapy for malignant liver tumors: an initial experience. J Radiat Res. 2016;57:174-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Peiffert D, Baumann AS, Marchesi V. Treatment of hepatic metastases of colorectal cancer by robotic stereotactic radiation (Cyberknife ®). J Visc Surg. 2014;151 Suppl 1:S45-S49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 17. | Dávila Fajardo R, Lekkerkerker SJ, van der Horst A, Lens E, Bergman JJ, Fockens P, Bel A, van Hooft JE. EUS-guided fiducial markers placement with a 22-gauge needle for image-guided radiation therapy in pancreatic cancer. Gastrointest Endosc. 2014;79:851-855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Ammar T, Coté GA, Creach KM, Kohlmeier C, Parikh PJ, Azar RR. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc. 2010;71:1204-1210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178-1184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Patel A, Khalsa B, Lord B, Sandrasegaran K, Lall C. Planting the seeds of success: CT-guided gold seed fiducial marker placement to guide robotic radiosurgery. J Med Imaging Radiat Oncol. 2013;57:207-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Kim JH, Hong SS, Kim JH, Park HJ, Chang YW, Chang AR, Kwon SB. Safety and efficacy of ultrasound-guided fiducial marker implantation for CyberKnife radiation therapy. Korean J Radiol. 2012;13:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Shirato H, Harada T, Harabayashi T, Hida K, Endo H, Kitamura K, Onimaru R, Yamazaki K, Kurauchi N, Shimizu T. Feasibility of insertion/implantation of 2.0-mm-diameter gold internal fiducial markers for precise setup and real-time tumor tracking in radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:240-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 290] [Cited by in F6Publishing: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Ohta K, Shimohira M, Sasaki S, Iwata H, Nishikawa H, Ogino H, Hara M, Hashizume T, Shibamoto Y. Transarterial Fiducial Marker Placement for Image-guided Proton Therapy for Malignant Liver Tumors. Cardiovasc Intervent Radiol. 2015;38:1288-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Brook OR, Gourtsoyianni S, Mendiratta-Lala M, Mahadevan A, Siewert B, Sheiman RR. Safety profile and technical success of imaging-guided percutaneous fiducial seed placement with and without core biopsy in the abdomen and pelvis. AJR Am J Roentgenol. 2012;198:466-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Valentine K, Cabrera T, Roberge D. Implanting metal fiducials to guide stereotactic liver radiation: McGill experience and review of current devices, techniques and complications. Technol Cancer Res Treat. 2014;13:253-258. [PubMed] [Cited in This Article: ] |
| 28. | Sotiropoulou E, Stathochristopoulou I, Stathopoulos K, Verigos K, Salvaras N, Thanos L. CT-guided fiducial placement for cyberknife stereotactic radiosurgery: an initial experience. Cardiovasc Intervent Radiol. 2010;33:586-589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, Navarria P, Fogliata A, Tomatis S, D’Agostino G. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol. 2015;141:1301-1309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Okabe T, Kimura T, Nagata Y. [Stereotactic body radiotherapy]. Gan To Kagaku Ryoho. 2014;41:2543-2545. [PubMed] [Cited in This Article: ] |
| 31. | Casamassima F, Cavedon C, Francescon P, Stancanello J, Avanzo M, Cora S, Scalchi P. Use of motion tracking in stereotactic body radiotherapy: Evaluation of uncertainty in off-target dose distribution and optimization strategies. Acta Oncol. 2006;45:943-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Machiels M, van Hooft J, Jin P, van Berge Henegouwen MI, van Laarhoven HM, Alderliesten T, Hulshof MC. Endoscopy/EUS-guided fiducial marker placement in patients with esophageal cancer: a comparative analysis of 3 types of markers. Gastrointest Endosc. 2015;82:641-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Fuccio L, Lami G, Guido A, Fabbri C. EUS-guided gold fiducial placement and migration rate. Gastrointest Endosc. 2014;80:533-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |