Summary
Abstract
Rivaroxaban (Xarelto®), an oral oxazolidinone-based anticoagulant, is a potent, selective direct inhibitor of factor Xa that is used in the prevention of venous thromboembolism (VTE) in adult patients after total hip replacement (THR) or total knee replacement (TKR) surgery. In four large, clinical trials, oral rivaroxaban was more effective than subcutaneous enoxaparin in preventing postoperative VTE in patients undergoing THR or TKR surgery. Notably, the superior efficacy of rivaroxaban was achieved with a low but not significant increase in the incidence of major bleeding episodes. In addition, preliminary pharmacoeconomic analyses in several countries indicate that rivaroxaban is a cost-effective treatment strategy versus enoxaparin. Although the position of rivaroxaban relative to other therapies remains to be fully determined, it is an effective emerging option for the prevention of VTE following THR and TKR.
Pharmacological Properties
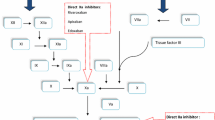
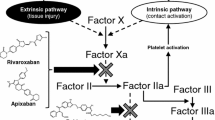
Rivaroxaban is a potent oral direct inhibitor of the serine endopeptidase factor Xa and inhibits both free factor Xa and factor Xa bound in the prothrombinase complex. The potency of factor Xa inhibition occurs primarily as a result of rivaroxaban binding with high selectivity to the S1 and S4 pockets of the serine endopeptidase.
In healthy volunteers and in orthopaedic surgery patients, rivaroxaban prophylaxis dose-dependently inhibited factor Xa and prolonged prothrombin (PT) and activated partial thromboplastin times confirming data from animal models. Plasma rivaroxaban concentrations correlated with both factor Xa inhibition and PT, following a maximum effect and linear model, respectively. Rivaroxaban prophylaxis did not prolong the corrected QT interval using the Fridericia formula.
Rivaroxaban pharmacokinetics following oral administration are best described by a one-compartment model and are characterized by rapid absorption (peak plasma concentration reached within 2–4 hours) and high bioavailability (80–100%). The apparent volume of distribution of rivaroxaban at steady state is ≈50 L. Rivaroxaban can be classified as a low-clearance drug, with a mean apparent oral clearance rate in orthopaedic surgery patients of 5.5 L/h on the day of surgery, increasing to 7.5 L/h at steady state, and mean terminal half-life of between 7 and 11 hours.
Approximately two-thirds of an administered rivaroxaban dose is metabolized via cytochrome P450 (CYP) enzymes (CYP3A4 and CYP2J2) and CYP-independent mechanisms, with one-third excreted as unchanged drug in the urine. The metabolized fraction of the drug is eliminated in approximately equal amounts via the renal and faecal routes.
Therapeutic Efficacy
In four large, randomized, double-blind (double-dummy), multicentre, phase III trials, known as RECORD1, 2, 3 and 4 studies, oral rivaroxaban 10 mg once daily regimens were more effective than subcutaneous enoxaparin at preventing postoperative VTE in patients undergoing THR or TKR surgery. Rivaroxaban was shown to be superior to enoxaparin regimens, with significantly lower incidences of the primary endpoint, total VTE (composite of deep vein thrombosis [DVT], nonfatal pulmonary embolism [PE] or death from any cause) observed in all four studies. For example, in the largest study (RECORD1), the primary endpoint occurred in 1.1% of rivaroxaban recipients and 3.7% of enoxaparin recipients.
Furthermore, a significantly lower incidence of the main secondary endpoint, major VTE (composite of proximal DVT, nonfatal PE or death from VTE), was observed with rivaroxaban regimens compared with enoxaparin regimens in RECORD1, 2 and 3. In a pooled analysis of the four RECORD trials, rivaroxaban regimens significantly reduced the incidence of the composite endpoint of symptomatic VTE and death compared with enoxaparin regimens, both over the total study duration and during the enoxaparin-controlled period.
Tolerability
Generally similar bleeding events were observed in the individual RECORD trials, with no significant between-group differences in major bleeding events, on-treatment non-major bleeding events and any on-treatment, bleeding events with rivaroxaban and enoxaparin treatments. Bleeding events were the most frequently reported adverse events associated with rivaroxaban prophylaxis across the four RECORD trials.
The most common adverse events (apart from bleeding) during rivaroxaban treatment across the RECORD trials were pyrexia, vomiting, nausea and constipation, and these events occurred at a similar frequency to that with enoxaparin treatment. Generally similar incidences of elevated liver enzymes were observed in rivaroxaban recipients compared with enoxaparin recipients across the four RECORD trials.
Pharmacoeconomic Considerations
In several modelled cost-effectiveness analyses conducted from a Canadian, Spanish, US and UK healthcare payer perspective, rivaroxaban was predicted to be cost effective relative to enoxaparin. Cost effectiveness was demonstrated irrespective of surgical procedure (THR or TKR), duration of treatment or dosage regimen.







Similar content being viewed by others
References
Hyers TM. Management of venous thromboembolism: past, present, and future. Arch Intern Med 2003; 163(7): 759–68
Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition). Chest 2008 Jun; 133 (6 Suppl.): 381–453S
Dalen JE, Paraskos JA, Ockene IS, et al. Venous thromboembolism: scope of the problem. Chest 1986 May; 89 (5 Suppl.): 370–433S
Department of Health. Report of the independent expert working group on the prevention of venous thromboembolism in hospitalised patients [online]. Available from URL: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_073944 [Accessed 2009 Mar 2]
Dahl OE. Orthopaedic surgery as a model for drug development in thrombosis. Drugs 2004; 64 Suppl. 1: 17–25
Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125(1): 1–7
Eriksson BI, Quinlan DJ, Weitz JI, et al. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet 2009; 48(1): 1–22
Eriksson BI, Quinlan DJ. Oral anticoagulants in development: focus on thromboprophylaxis in patients undergoing orthopaedic surgery. Drugs 2006; 66(11): 1411–29
Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939: an oral, direct factor Xa inhibitor. J Thromb Haemost 2005 Mar; 3(3): 514–21
Depasse F, Busson J, Mnich J, et al. Effect of BAY 59-7939: a novel, oral direct factor Xa inhibitor on clot-bound factor Xa activity in vitro [abstract no. P1 104]. J Thromb Haemost 2005 Aug 6-2005; 3 Suppl. 1: P1104
Laux V, Perzborn E, Kubitza D, et al. Preclinical and clinical characteristics of rivaroxaban: a novel, oral, direct factor Xa inhibitor. Semin Thromb Hemost 2007 Jul; 33(5): 515–23
Roehrig S, Straub A, Pohlmann J, et al. Discovery of the novel antithrombotic agent 5-chloro-N-((5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl) phenyl]-1,3-oxazolidin-5-yl methyl) thiophene-2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J Med Chem 2005 Sep 22; 48(19): 5900–8
Bayer Schering Pharma. Xarelto® (rivaroxaban 10mg tablets): EU summary of product characteristics [online]. Available from URL: http://www.xarelto.com/html/downloads/Xarelto_Summary_of_Product_Characteristics_30Sept2008.pdf [Accessed 2008 Nov 22]
Perzborn E, Lange U. Rivaroxaban: an oral, direct factor Xa inhibitor inhibits tissue factor-mediated platelet aggregation [abstract no. P-W-642]. J Thromb Haemost 2007 Aug; 5 Suppl. 2: P–W–642
Wong PC, Crain E, Watson C, et al. Comparative antithrombotic and antihemostatic effects of the direct factor Xa inhibitors, apixaban and rivaroxaban, and the direct thrombin inhibitors, dabigatran and lepirudin, in rabbit models of venous thrombosis and bleeding time [abstract no. 3025]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Francisco (CA)
Kubitza D, Becka M, Voith B, et al. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 2005 Oct; 78(4): 412–21
Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 — an oral, direct factor Xa inhibitor — after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005 Dec; 61(12): 873–80
Mueck W, Becka M, Kubitza D, et al. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban — an oral, direct factor xa inhibitor — in healthy subjects. Int J Clin Pharmacol Ther 2007 Jun; 45(6): 335–44
Mueck W, Borris LC, Dahl OE, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost 2008 Sep; 100(3): 453–61
Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban — an oral, direct factor Xa inhibitor — in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47(3): 203–16
Graff J, von Hentig N, Misselwitz F, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on platelet-induced thrombin generation and prothrombinase activity. J Clin Pharmacol 2007 Nov; 47(11): 1398–407
Gerotziafas GT, Elalamy I, Depasse F, et al. In vitro inhibition of thrombin generation, after tissue factor pathway activation, by the oral, direct factor Xa inhibitor rivaroxaban [letter]. J Thromb Haemost 2007 Apr; 5(4): 886–8
Walenga JM, Prechel M, Jeske WP, et al. Rivaroxaban — an oral, direct factor Xa inhibitor — has potential for the management of patients with heparin-induced thrombocytopenia. Br J Haematol 2008 Jul 30; 143: 92–9
Kubitza D, Becka M, Mueck W, et al. Rivaroxaban (BAY 59-7939) — an oral, direct factor Xa inhibitor — has no clinically relevant interaction with naproxen. Br J Clin Pharmacol 2007 Apr; 63(4): 469–76
Kubitza D, Becka M, Mueck W, et al. Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban — an oral, direct factor Xa inhibitor — are not affected by aspirin. J Clin Pharmacol 2006 Sep; 46(9): 981–90
Kubitza D, Becka M, Mueck W, et al. Co-administration of rivaroxaban — a novel, oral, direct factor Xa inhibitor — and clopidogrel in healthy subjects [abstract no. 1272]. Eur Heart J 2007 Sep; 28 (Abstr. Suppl.): 189
Kubitza D, Mueck W, Becka M. Randomized, double-blind, crossover study to investigate the effect of rivaroxaban on QT-interval prolongation. Drug Saf 2008; 31(1): 67–77
Kubitza D, Becka M, Zuehlsdorf M, et al. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol 2007 Feb; 47(2): 218–26
Kubitza D, Becka M, Zuehlsdorf M, et al. Effects of single-dose BAY 59-7939 — an oral, direct factor Xa inhibitor — in subjects with extreme body weight [abstract no. 1872]. Blood 2005 Nov 16; 106(11): 532
Halabi A, Maatouk H, Klause N, et al. Effects of renal impairment on the pharmacology of rivaroxaban — an oral, direct, factor Xa inhibitor [abstract no. 913]. Blood 2006 Nov 1; 108(11): 274
Kubitza D, Becka M, Mueck W, et al. The effect of age and gender on the pharmacology and safety of the oral, direct factor Xa inhibitor rivaroxaban [abstract no. P-T-628]. 21st Congress of the International Society on Thrombosis and Haemostasis; 2007 Jul 6–12; Geneva
Kubitza D, Becka M, Mueck W, et al. The effect of extreme age, and gender on the pharmacology and tolerability of rivaroxaban: an oral, direct factor Xa inhibitor. Blood 2006; 108(11): 271–2
Kubitza D, Becka M, Voith B, et al. Effect of enoxaparin on the safety, tolerability, pharmacodynamics, and pharmacokinetics of BAY 59-7939: an oral, direct factor Xa inhibitor [abstract no. P1704]. J Thromb Haemost 2005; 3 Suppl. 1: P1704
Kubitza D, Becka M, Zuehlsdorf M, et al. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol 2006 May; 46(5): 549–58
Bayer Inc. Xarelto® (rivaroxaban 10 mg tablets): product monograph [online]. Available from URL: http://bayer.ca/files/XARELTO-PM-ENG-10SEP2008-119111.pdf?# [Accessed 2009 Feb 25]
Lang D, Freudenberger C, Weinz C. In vitro metabolism of rivaroxaban, an oral, direct factor Xa inhibitor, in liver microsomes and hepatocytes of rats, dogs, and humans. Drug Metab Dispos 2009 Feb 5; 37(5): 1046–55
Weinz C, Schwarz T, Kubitza D, et al. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos 2009 Feb 5; 37(5): 1056–64
Kubitza D, Becka M, Zuehlsdorf M, et al. No interaction between the novel, oral, direct factor Xa inhibitor BAY 59-7939 and digoxin [abstract no. 11]. J Clin Pharmacol 2006; 46: 702
Eriksson BI, Borris L, Dahl OE, et al. Oral, direct factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 2006 Jan; 4(1): 121–8
Eriksson BI, Borris LC, Dahl OE, et al. A once-daily, oral, direct factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation 2006 Nov 28; 114(22): 2374–81
Turpie AG, Fisher WD, Bauer KA, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement: a phase II dose-ranging study. J Thromb Haemost 2005 Nov; 3(11): 2479–86
Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008 Jun 26; 358(26): 2765–75
Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008 Jun 26; 358(26): 2776–86
Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. Lancet 2009 May 16; 373(9676): 1673–80
Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008 Jul 5; 372(9632): 31–9
Turpie AG, Lassen MR, Kakkar AK. A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopaedic surgery: effect on symptomatic venous thromboembolism, death, and bleeding [abstract no. 36]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Fransisco (CA)
Bauer KA, Homering M, Berkowitz SD, et al. Effects of age, weight, gender and renal function in a pooled analysis of four phase III studies of rivaroxaban for prevention of venous thromboembolism after major orthopaedic surgery [abstract no. 436]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Francisco (CA)
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Rivaroxaban for the prophylaxis of deep vein thrombosis (DVT) and pulmonary embolism (PE) in patients undergoing hip or knee replacement surgery. FDA advisory committee briefing book [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4418b1-03-Johnson_Johnson.pdf [Accessed 2009 Jul 17]
Eriksson BI, Turpie AG, Lassen MR, et al. A pooled analysis of four pivotal studies of rivaroxaban for the prevention of venous thromboembolism after orthopaedic surgery: effects of specified co-medications [abstract no. 1986]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Fransisco (CA)
Diamantopoulos A, Forster F, Lees M, et al. Cost-effectiveness of rivaroxaban versus enoxaparin for thromboprophylaxis after total hip replacement in the UK [abstract no. PHC9]. Value Health 2008 Nov; 11 (6): A534. Plus poster presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 11th Annual European Congress; 2008 Nov 8–11; Athens
Diamantopoulos A, Forster F, Brosa M, et al. Cost-effectiveness of rivaroxaban versus enoxaparin for thromboprophylaxis after total hip replacement in Spain [abstract no. PHC6]. Value Health 2008 Nov; 11 (6): A533. Plus poster presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 11th Annual European Congress; 2008 Nov 8–11; Athens
Kwong L, Diamantopoulos A, Forster F, et al. Will rivaroxaban be cost-effective for prevention of venous thromboembolism after total hip replacement in US patients? [abstract no. 1290 plus poster]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Francisco (CA)
Wells P, Diamantopoulos A, Forster F, et al. Cost-effectiveness of rivaroxaban as VTE prophylaxis after total hip replacement in Canada [abstract no. 1291 plus poster]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Francisco (CA)
Diamantopoulos A, Forster F, Brosa M, et al. Cost-effectiveness of rivaroxaban versus enoxaparin for thromboprophylaxis after total knee replacement in the UK and Spain [abstract no. PHC8]. Value Health 2008 Nov; 11 (6): A533-4. Plus poster presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 11th Annual European Congress; 2008 Nov 8–11; Athens
National Institute for Health and Clinical Excellence. NICE technology appraisal guidance 170. Rivaroxaban for the prevention of venous thromboembolism after total hip or total knee replacement in adults [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/TA170Guidance.pdf [Accessed 2009 Apr 24]
National Institute for Health and Clinical Excellence. Costing statement: rivaroxaban for the prevention of venous thromboembolism after total hip or total knee replacement in adults [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=download&o=43807 [Accessed 2009 Apr 30]
Johnson and Johnson Pharmaceutical Research and Development L.L.C. Johnson and Johnson Pharmaceutical Research and Development, L.L.C. submits new drug application to FDA for rivaroxaban [media release]. 2008 Jul 30 [online]. Available from URL: http://www.jnjpharmarnd.com [Accessed 2009 Feb 2]
Eikelboom JW, Karthikeyan G, Fagel N, et al. American Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients? Chest 2009 Feb; 135(2): 513–20
American Academy of Orthopaedic Surgeons clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. Adopted by the American Academy of Orthopedic Surgeons board of directors May 2007 [online]. Available from URL: http://www.aaos.org/research/guidelines/PE_guideline.pdf. [Accessed 2009 May 22]
National Institute for Health and Clinical Excellence. Venous thromboembolism. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in inpatients undergoing surgery. NICE clinical guideline 46 [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=download&o=30469 [Accessed 2009 Feb 2]
Nicolaides AN, Fareed J, Kakkar AK. Prevention and treatment of venous thromboembolism: international consensus statement (guidelines according to scientific evidence). Int Angiol 2006 Jun; 25(2): 101–61
Warwick D, Friedman RJ, Agnelli G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br 2007 Jun; 89(6): 799–807
Amin AN, Stemkowski S, Lin J, et al. Preventing venous thromboembolism in US hospitals: are surgical patients receiving appropriate prophylaxis? [letter]. Thromb Haemost 2008 Apr; 99(4): 796–7
Reynolds NA, Perry CM, Scott LJ. Fondaparinux sodium: a review of its use in the prevention of venous thromboembolism following major orthopaedic surgery. Drugs 2004; 64(14): 1575–96
Mori S, Matsuura A, Rama Prasad YV, et al. Studies on the intestinal absorption of low molecular weight heparin using saturated fatty acids and their derivatives as an absorption enhancer in rats. Biol Pharm Bull 2004 Mar; 27(3): 418–21
European Medicines Agency. European public assessment report. Pradexa [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/pradaxa/H-829-PI-en.pdf [Accessed 2009 Feb 25]
Boehringer Ingelheim Canada Ltd. Pradax™ (dabigatran etexilate capsules 75mg and 110mg): product monograph [online]. Available from URL: http://www.boehringeringelheim.ca/ethical/products/pm/Pradax_Capsules.pdf [Accessed 2009 Apr 24]
Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol 2008 Mar; 28(3): 380–6
Bounameaux H. The novel anticoagulants: entering a new era. Swiss Med Wkly 2007 Feb 7; 139(5–6): 60–4
Carter NJ, McCormack PL, Plosker GL. Enoxaparin: a review of its use in ST-segment elevation myocardial infarction. Drugs 2008; 68(5): 691–710
Lu G, DeGuzman FR, Lakhotia S, et al. Recombinant antidote for reversal of anticoagulation by factor Xa inhibitors [abstract no. 983]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Francisco (CA)
Gruber A, Marzec UM, Buetehorn U, et al. Potential of activated prothrombin complex concentrate and activated factor VII to reverse the anticoagulant effects of rivaroxaban in primates [abstract no. 3825]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Francisco (CA)
Fareed J, Ma Q, Florian M, et al. Differentiation of low-molecular-weight heparins: impact on the future of the management of thrombosis. Semin Thromb Hemost 2004 Feb; 30 Suppl. 1: 89–104
Diamantopoulos A, LeReun C, Rasul F, et al. Indirect comparisons of rivaroxaban vs alternative prophylaxis for the prevention of venous thromboembolism in patients undergoing total hip or total knee replacement [abstract no. 1292]. 50th American Society of Hematology Annual Meeting and Exposition; 2008 Dec 6–9; San Fransisco (CA)
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: S. Haas, Institute for Experimental Oncology and Therapy Research, Technical University Munich, Munich, Germany; J. Harenberg, IV Department of Medicine, University Hospital Mannheim, Mannheim, Germany; T.M. Hyers, Department of Internal Medicine, St. Louis University, St. Louis, Missouri, USA; M.M. Samama, Department of Biological Haematology, Hotel-Dieu Hospital, Paris, France; A.G. Turpie, Department of Medicine, McMaster University, Hamilton, Ontario, Canada.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘rivaroxaban’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘rivaroxaban’ and (‘venous thromboembolism’ or ‘orthopaedic surgery’). Searches were last updated on 11 August 2009.
Selection: Studies in patients who underwent total hip or knee replacement surgery who received rivaroxaban. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Rivaroxaban, venous thromboembolism, deep vein thrombosis, pulmonary embolism, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Duggan, S.T., Scott, L.J. & Plosker, G.L. Rivaroxaban. Drugs 69, 1829–1851 (2009). https://doi.org/10.2165/11200890-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11200890-000000000-00000