Abstract
Individuals who have been infected with varicella zoster virus (VZV) are at risk for developing herpes zoster and this risk appears to be related to a decline in VZV-specific cell-mediated immunity (CMI). Zostavax® (zoster vaccine) is a one-dose, high-potency, live, attenuated VZV vaccine that boosts VZV-specific CMI and this is its presumed mechanism of action. Zoster vaccine is registered in the EU for use in adults aged ≥50 years for the prevention of herpes zoster and herpes zoster-related postherpetic neuralgia.
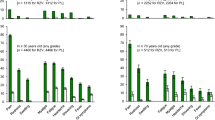
In the Shingles Prevention Study, a placebo-controlled trial in adults aged ≥60 years (n = 38 546), zoster vaccine led to a sustained boost of VZV-specific CMI. Over a mean herpes zoster surveillance period of 3.1 years, zoster vaccine reduced the herpes zoster-related burden of illness by 61%, reduced the incidence of herpes zoster by 51% and reduced the incidence of postherpetic neuralgia by 67%. Zoster vaccine recipients who developed herpes zoster had a shorter illness duration and severity than placebo recipients who developed herpes zoster. Zoster vaccine had continuing efficacy in a Shingles Prevention Study sub-population followed for 7 years post-vaccination. Zoster vaccine was generally well tolerated in older adults. While cost-effectiveness estimates in pharmaco-economic analyses varied widely according to vaccine and herpes zoster parameter cost/benefit estimates, an analysis from a UK perspective found a zoster vaccine immunization programme in adults aged 65 years to be cost effective. In older adults, the zoster vaccine has the potential to significantly reduce the herpes zoster burden of illness by decreasing the incidence of herpes zoster or reducing its severity.






Similar content being viewed by others
References
Johnson RW, Wasner G, Saddier P, et al. Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging 2008 Dec; 25(12): 991–1006
Araújo LQ, MacIntyre CR, Vujacich C. Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America. Herpes 2007; 14Suppl. 2: 40A–4A
Schmader K, Gnann Jr JW, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis 2008 Mar 1; 197Suppl. 2: S207–15
Van Hoek AJ, Gay N, Melegaro A. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009; 27(9): 1454–67
Cohen JI. Strategies for herpes zoster vaccination of immunocompromised patients. J Infect Dis 2008; 197Suppl. 2: s237-41
Johnson RW, Rice ASC. Pain following herpes zoster: the influence of changing population characteristics and medical developments. Pain 2007; 128(1–2): 3–5
Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008; 197(6): 825–35
Gauthier A, Breuer J, Carrington D, et al. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 2009 Jan; 137(1): 38–47
Schmader K. Herpes zoster in older adults. Clin Infect Dis 2001; 32(10): 1481–6
Li Q, Chen N, Yang J, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Databse Syst Rev 2009 Apr 15; (2): CD006866
Zin CS, Nissen LM, Smith MT, et al. An update on the pharmacological management of post-herpetic neuralgia and painful diabetic neuropathy. CNS Drugs 2008; 22(5): 417–42
Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine 2006; 24(9): 1308–14
European Medicines Agency. Zostavax: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/zostavax/H-674-PI-en.pdf [Accessed 2009 Jul 1]
Merck. Varivax®: prescribing information [online]. Available from URL: http://www.merck.com/product/usa/pi_circulars/v/varivax_pi.pdf [Accessed 2009 Mar 4]
Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352(22): 2271–84 plus supplementary material available from http://content.nejm.org/cgi/content/full/352/22/2271/DC1
Schmader K, Oxman MN, Levin M, et al. Persistence of zoster vaccine efficacy [abstract no. G-409 plus poster]. 48th Annual Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washington, DC
Oxman MN, Williams HM, Levin MJ, et al. The impact of age on the efficacy of zoster vaccine [poster]. 43rd Annual Meeting of the Infectious Diseases Society of America; 2005 Oct 6–8; San Francisco (CA)
Oxman MN, Williams HM, Levin MJ, et al. Efficacy of zoster vaccine according to dermatome region [abstract no.2699 plus poster]. 45th Annual Meeting of the Inter-science Conference on Antimicrobial Agents and Chemotherapy; 2005 Dec 15–18; Washington, DC
Schmader K, Saddier P, Johnson G, et al. The effect of zoster vaccine on interference of herpes zoster with activities of daily living (ADL) [poster]. 43rd Annual Meeting of the Infectious Diseases Society of America; 2005 Oct 6–8; San Francisco (CA)
Oxman MN, Levin MJ. Vaccination against herpes zoster and postherpetic neuralgia (Shingles Prevention Study Group). J Infect Dis 2008 Mar 1; 197Suppl. 2: 228–36
Robinson DM, Perry CM. Zoster vaccine live (Oka/Merck). Drugs Aging 2006; 23(6): 525–31
Levin MJ, Murray M, Rotbart HA, et al. Immune response of elderly individuals to a live attenuated varicella vaccine. J Infect Dis 1992; 166: 253–9
Levin MJ, Murray M, Zerbe GO, et al. Immune responses of elderly persons 4 years after receiving a live attenuated varicella vaccine. J Infect Dis 1994 Sep; 170: 522–6
Levin MJ, Barber D, Goldblatt E, et al. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis 1998 Nov; 178Suppl. 1: 109–12
Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis 2009; 200(7): 1068–77
Gilderman LI, Lawless JF, Nolen TM, et al. A double-blind, randomized, controlled, multicenter safety and immuno-genicity study of a refrigerator-stable formulation of Zostavax. Clin Vaccine Immunol 2008; 15(2): 314–9
Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007; 55(10): 1499–507
MacIntyre CR, Egerton T, McCaughey M, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old [abstract no.G-399d plus poster]. 48th Annual Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washington, DC
Sutradhar SC, Wang WW, Schlienger K, et al. Comparison of the levels of immunogenicity and safety of Zostavax in adults 50 to 59 years old and adults 60 years and older. Clin Vaccine Immunol 2009 May; 16(5): 646–52
Mills R, Tyring S, Lawless J et al. Safety, tolerability and immunogenicity of zoster vaccine in subjects with a history of herpes zoster (HZ) [abstract no. G-407 plus poster]. 48th Annual Meeting of the Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Meeting of the Infectious Diseases Society of America; 2008 Oct 25–28; Washington, DC
Schmader KE, Bobrove AM, Levin MJ, et al. Immunogenicity & safety of varicella-zoster virus (VZV) vaccine administered to older adults with or without diabetes mellitus (DM) or chronic obstructive pulmonary disease (COPD) [abstract no. D26 plus poster]. 2006 American Geriatrics Society Annual Meeting; 2006 May 3–7; Chicago (IL)
Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med 2006; 145(5): 317–25
Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25(49): 8326–37
Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007 May 15; 44(10): 1280–8
Brisson M, Pellissier JM, Camden S, et al. The potential cost-effectiveness of vaccination against herpes zoster and post-herpetic neuralgia. Hum Vaccin 2008; 4(3): 238–45
Najafzadeh M, Marra CA, Galanis E, et al. Cost effectiveness of herpes zoster vaccine in Canada. Pharmacoeconomics 2009; 27(12): 991–1004
Merck. Zostavax®: prescribing information [online]. Available from URL: http://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi.pdf [Accessed 2009 Mar 4]
Merck Frosst. Zostavax™ approved in Canada: the first and only vaccine for the prevention of shingles [media release] 2008 Aug 28 [online]. Available from URL: http://www.merckfrosst.com
Brisson M. Estimating the number needed to vaccinate to prevent herpes zoster-related disease, health care resource use and mortality. Can J Pub Health 2008; 99(5): 383–6
Macaladad N, Marcano T, Guzman M, et al. Safety and immunogenicity of a zoster vaccine in varicella-zoster virus seronegative and low-seropositive healthy adults. Vaccine 2007 Mar 1; 25(11): 2139–44
Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008 Jun; 57(RR-5): 1–30
Chidiac C, Bruxelle J, Daures J-P, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis 2001 Jul 1; 33: 62–9
Levine MJ, Ellison MC, Zerbe GO, et al. Comparison of a live attenuated and an inactivated varicella vaccine to boost the varicella-specific immune response in seropositive people 55 years of age and older. Vaccine 2000; 18: 2915–20
Gnann Jr JW. Vaccination to prevent herpes zoster in older adults. J Pain 2008 Jan; 9(1 Suppl. 1): S31–6
Lieu TA, Ortega-Sanchez I, Ray GT, et al. Community and patient values for preventing herpes zoster. Pharmacoeconomics 2008 Jan; 26(3): 235–49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanford, M., Keating, G.M. Zoster Vaccine (Zostavax®). Drugs Aging 27, 159–176 (2010). https://doi.org/10.2165/10489140-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/10489140-000000000-00000