Article contents
A reinvestigation of holotype wadalite from Tadano, Fukushima Prefecture, Japan
Published online by Cambridge University Press: 15 May 2018
Abstract
A reinvestigation of the type specimen of wadalite, ideally Ca12Al10Si4O32Cl6, from Tadano, Fukushima Prefecture, Japan, reveals that it has well-defined chemical sector zoning. The mineral occurs in skarn xenoliths in two-pyroxene andesite and forms tris-tetrahedral crystals up to 1 mm in size. The mineral is cubic, I¯43d, with a = 12.009(2) Å, V = 1731.8(8) Å3 and Z = 2. The zoning is divided into two sectors, {21¯1} and {211}, on the basis of back-scattered-electron images. The {21¯1} sector is more enriched in Fe3+, Si, Mg and Cl, and depleted in Al than the {211} sector. The empirical formulae yielded by the averaged compositions for the {21¯1} and {211} sectors are: Ca12.01(Al7.88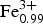 $\hbox{Fe}_{{\rm 0}{\rm. 99}}^{{\rm 3 +}} $Si4.51Mg0.56Ti0.05)Σ13.99O32.22Cl5.55 and Ca12.05(Al8.42
$\hbox{Fe}_{{\rm 0}{\rm. 99}}^{{\rm 3 +}} $Si4.51Mg0.56Ti0.05)Σ13.99O32.22Cl5.55 and Ca12.05(Al8.42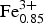 $\hbox{Fe}_{{\rm 0}{\rm. 85}}^{{\rm 3 +}} $Si4.20Mg0.44Ti0.04)Σ13.95O32.19Cl5.38, respectively. Compositional variations in the wadalite grains suggest that the Si contents are controlled mainly by the substitution T(Al,Fe3+) + W□ ↔ TSi + W(Cl,F). The presence of sector zoning suggests that the wadalite grew during rapid non-equilibrium crystallization.
$\hbox{Fe}_{{\rm 0}{\rm. 85}}^{{\rm 3 +}} $Si4.20Mg0.44Ti0.04)Σ13.95O32.19Cl5.38, respectively. Compositional variations in the wadalite grains suggest that the Si contents are controlled mainly by the substitution T(Al,Fe3+) + W□ ↔ TSi + W(Cl,F). The presence of sector zoning suggests that the wadalite grew during rapid non-equilibrium crystallization.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2018
Footnotes
Associate Editor: Ian Graham
References
- 1
- Cited by




