Abstract
Nanosensors are sensing devices with at least one of their sensing dimensions being up to100 nm. In the field of nanotechnology, nanosensors are instrumental for (a) detecting physical and chemical changes, (b) monitoring biomolecules and biochemical changes in cells, and (c) measuring toxic and polluting materials presented in the industry and environment. Nanosensors can be classified according to their energy source, structure and applications. The nanostructured materials used in manufacturing of nanosensors are such as: nanoscale wires (capability of high detection sensitivity), carbon nanotubes (very high surface area and high electron conductivity), thin films, metal and metal oxides nanoparticles, polymer and biomaterials. The aim of this review is to provide an overview of all classifications of nanosensors, showing the characteristcs and functioning mechanisms among the various categories.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
A sensor is a device which detects a variable quantity, usually electronically, and converts the measurement into specific signals. The most important requirements of sensors are diversity, sensitivity, accuracy of information extracted, selectivity, and stability.1 Nanosensors are applied for monitoring physical and chemical phenomena in regions difficult to reach, detecting biochemicals in cellular organelles, measuring nanoscopic particles in the industry and environment. Chemical detectors are used in applications for:
- Industrial: leak detection, food quality control
- Environmental: quality of air and water
- Military: anti-terrorism applications
- Aerospace: chemical analysis of soil and atmospheric constituents.2
Jung et al.3 developed solid state potentiometric sensors based on a Nafion electrolyte covered with Pt-C electrodes for environmental monitoring of hydrogen gas. These sensors were characterized by good sensitivity, short response time, wide linear range, and long-term stability greater than 3 months.
Another important application for environmental issues, is the ability of a chemiresistor sensor with a polyaniline active layer to detect insect infestation. The sensor detected various volatile organic compounds produced by plants, emitted as a defense mechanism when attacked by herbivores. Detecting for these phytochemicals enables detecting insect infestation at early stages.4
The ability to detect important molecules, such as disease-related metabolites, proteins, nucleic acids, pathogens, and cells is important not only for disease diagnosis in the clinical setting and health technology but also for industrial, environmental and agricultural research development.5
Nanosensors are sensing devices with at least one of their sensing dimensions up to 100 nm. The nanostructured materials used in production of nanosensors are such as: nanoscale wires (capability of high detection sensitivity), carbon nanotubes (very high surface area), thin films, nanoparticles, and polymer nanomaterials.6
Carbon-based nanomaterials have wide range of applications including monitoring heavy metal ions, gas molecules, food additives, antibodies, and toxic pesticides, as well as bioimaging.7 On the other hand, the superior physicochemical, spectral and optical characteristics of noble metal nanoparticles have allowed the synthesizing of new biosensors.8 Metal oxide nanowires are promising class of sensing nanomaterials due to their easy fabrication techniques and chemical stability.9
The following properties and related topics should be considered during manufacturing of nanomaterials.
- Attain the optimal electrochemical active sites, by controlling size and shape of nanomaterials during synthesizing process.
- Improve the specificity and stability, by optimization of bi- or tri-metallic nanomaterials
- Provide high specificity with analytes by the discovery of functional molecules.
- Design novel nanomaterials with exceptional selectivity by making a correlation between composition, structure, and surface reactivity of nanomaterials.
- Improve the electrochemical properties through the discovery of high conductive, chemical and mechanical stable and great surface area of substrate materials.
- Offer great platform for the unconventional electrocatalytic properties by improving the sensitivity and stability of the nanosensors.10
Most review articles on nanosensors are focused on specific types of sensors, such as nanobiosensors, optical nanosensors and magnetic nanosensors. The aim of this review is to provide an overview of all types and classifications of nanosensors, comparing the chracteristics and main differences among the various categories, in addition to different nanomaterials used.
Classification of Nanosensors
As illustrated in Table I11 and Fig. 112 nanosensors can be classified according to its energy source, structure and applications.
- 1.According to energy source: In this case the nanosensors are classified as (i) active nanosensors that need an energy source such as a thermistor, and (ii) passive nanosensors where no energy source is needed, such as a thermocouple, and piezoelectric sensor.
- 2.Classification based on structure: Four types of sensors are classified based on structure namely; (i) optical nanosensors, (ii) electromagnetic nanosensors, and (iii) mechanical and/or vibrational nanosensors.13
- 3.Classification based on application:Four types of sensors are identified based on application; (i) chemical sensors, (ii) deployable nanosensors, (iii) electrometers, (iv) biosensors.14
Table I. Classification of nanosensors.11
| Stimuli | Properties |
|---|---|
| Mechanical | Position, acceleration, stress, strain, force, pressure, mass, density, viscosity, moment, torque acoustic wave amplitude, phase, polarization, velocity |
| Optical | Absorbance, reflectance, fluorescence, luminescence, refractive index, light scattering |
| Thermal | Temperature, flux, thermal conductivity, specific heat |
| Electrical | Charge, current, potential, dielectric constant, conductivity |
| Magnetic | Magnetic field, flux, permeability |
| Chemical | Components (identities, concentrations, states) |
| Biological | Biomass (identities, concentrations, states) |
Figure 1. Classification of nanosensors.12
Download figure:
Standard image High-resolution imageOptical nanosensors
Optical sensors are capable of monitoring chemical analysis. They depends on optical properties of nanomaterials. They can be applied in different areas such as the chemical industry, biotechnology, medicine, environmental sciences, and human protection.
The first reported optical nanosensor was based on fluorescein which is trapped within a polyacrylamide nanoparticle, and was used for pH measurement.15 Basically, fluorescent sensors are particles including at least one binding component(s) and photoactive units(s).16,17 The luminescence phenomenon is a process by which a fluorophore absorbs light of a certain wavelength, which is followed by emission of a quantum of light with an energy corresponding to the energetic difference between the ground and stimulated states.18,19
The most basic type of optical nanosensor is that of a molecular fluorescent dye probe inside a cell reported by Sasaki et al.20 The advantage of this basic approach is to minimize the physical perturbation of the cell. However, a disadvantage of the free dye is the inherent dye-cell chemical interference as a result of protein binding, cell sequestration and toxicity. Another method is known as the labelled nanoparticles that consists of a reporter molecule attached to the outside of the nanoparticles.21,22 The major difference between the labelled nanoparticles and the free dye method is the solid state and fluid nature of the former and latter, respectively. Similar to the free dye, the labelled nanoparticles are freely flowing and the reporter molecules are in contact with the intracellular components. The outer-labelled particles sensors type of sensors have been used for intracellular sensing, but retain similar drawbacks of using the free fluorescent dyes because the signal is derived from receptor molecules not insulated from the cellular environment.23,24
Fiber optic nanosensors
Fiber optic nanosensors have the potential to analyze important cellular processes in vivo. The first optical fiber submicron nanosensor is attributed to Tan et al.25,26 The interaction between the target molecule (A) and the receptor (R) is designed to produce a physicochemical perturbation that can be converted into an electrical signal or other measurable signal:27–29
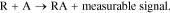
This measurable signal is then picked up by the optical probe and transmitted into the database. The disadvantages associated with the chemical interference of the color cell in the color free method are overcome due to spacing the optical fiber arm between the surrounding and sensitive area. Another advantage of the optical nanosensor is that the minimum level of invasion of been reached.
Electromagnetic nanosensors
There are two types of sensors under the category of electromagnetic nanosensors, based on their detection mechanisms:
- 1.Monitoring via electrical current measurement.
- 2.Monitoring via magnetism measurement.
Electrical current measurement
The advantage of this approach is the label-free methodology over the use of dyes. Geng et al.30 investigated the interaction between hydrogen sulfide gas molecules and gold nanoparticles. In each sensing cell, chromium electrode and gold electrode, source and drain. A typical gap-width of ca. 40–60 nm has been achieved between the two electrodes. Au nanoparticles are placed randomly over the gap area. The formation of sulfide shell, inhibits the "e" charge transfer from one nanoparticle to another, ie the so called hopping phenomena. By using the current and voltage across chromium and gold electrodes in the existence of an applied electrical field, the hopping of electrons was determined (Fig. 2).
Figure 2. Electric sensor detection of analytes via inhibition of electron hopping (a) before bonding, and (b) after bonding.30
Download figure:
Standard image High-resolution imageMagnetism measurement
These magnetic nanosensors have been designed to detect specific biomolecules such as; proteins, enzymatic activity, and pathogens (e.g., virus) with sensitivity in the low femtomolar range (0.5 ± 30 fmol). Magnetic nanosensors are composed of magnetic nanoparticles (iron oxide). When these magnetic nanoparticles bind to their intended molecular target, they form stable nanoasemblies. This leads to a corresponding decrease in the spin-spin relaxation time (T2) of surrounding water molecules, which consequently can be detected by magnetic resonance (NMR/MRI) techniques.31
Mechanical nanosensors
Mechanical nanosensors possess comparative advantages over optical nanosensors and electromagnetic nanosensors for the detection of nanoscale mechanical properties. There are many types of mechanical nanosensors such as CNT-based fluidic shear-stress sensors and the nano mechanical cantilever sensors. Binh et al.32 proposed the earliest mechanical nanosensor for monitoring the vibrational and elastic properties of a nanosphere attached to a tapered cantilever. The role of mechanical sensors is essential for application in nanodevices components and nano-scale subassemblies in microelectronic devices.
Classification of Sensors According its Applications
Chemical nanosensors
This type can be applied to analyze a single chemical or molecule. Several different optical chemical nanosensors were used for measuring some properties such as pH, and various ion concentrations.
Deployable nanosensors
This type is used in military or other forms of national security such as Sniffer STAR. It is characterized by a lightweight, portable chemical detection system that combines a nanomaterials for sample collection and a concentration with a micro electromechanical detector.
Electrometers
It consists of mechanical resonator, a detection electrode, and a gate electrode which are used to couple charge to the mechanical element.
Biosensors
It is one of the most commonly sensors used due to the possibilities of early cancer detection and detection of other various diseases. It can also be used to detect specific type of DNA.33 The biosensor can usually be considered a subset of chemical sensors because the transduction methods or the so called sensor platforms, are similar to those for chemical sensors.34
Within different kinds of developed bio sensing technologies, field-effect transistor (FET) have many advantages such as; ultra-sensitivity detection, mass production capability, and low-cost manufacturing.35 The major FET-based bio sensing devices are: ion sensitive field-effect transistor (ISFET), silicon nanowire, organic FET, graphene FET, and compound-semiconductor FET.36
Rai et al.37 studied the textile based wearable nano-biosensors. This type can detect neurological signals and identify anomalies for diagnosis of targeted neurological and cardiovascular disorders.
Biosensors-on-chip
Microfluidic biosensors (biosensors-on-chip) or (lap-on-chip) are essential for developing robust and cost effective point-of-care diagnostics.38 The integration of microfluidic and biosensor technologies provides the ability to merge chemical and biological components into a single platform and offers new approach for biosensing applications such as portability, disposability, real-time detection, unprecedented accuracies, and simultaneous analysis of different analytes in a single device.39 Das et al.40,41 used this technique for detection of nucleic acids (cfNAs), which are present at significant levels in the blood of cancer patients.
Nanomaterials Applied for Nanosensors
Recently, nanostructures from metal, metal oxide, carbon nanotubes, graphene, have been widely explored for chemiresistive sensing applications. The small size and high surface to volume ratio of nanomaterials provides several benefits for sensing over more than the traditional bulk films.
Metal and noble metals nanomaterials
Metal nanoparticles have unique physical and chemical properties which have been widely applied for many applications. Various metals such as Au, Pt, Pd, Ag, Cu, Co, including rare earth metals have been employed for sensing.10,42
Metal nanoparticles-based sensors provide a strong potential with increasing both sensitivity and selectivity via tuned signal amplifications. The design of the metal nanoparticles, bio-functionalized nanoparticles and nanocomposites have attracted research focused on nanosensors. Advanced numerous analytical methods were developed for environmental monitoring and food safety applications.43
Noble metals with outstandingly resistant to corrosion and oxidation even at elevated temperatures include the metals of groups VIIb, VIII and 1b of the second and third transition series of the periodic table i.e. rhodium (Rh), ruthenium (Ru), palladium (Pd), silver (Ag), osmium(Os), iridium (Ir), platinum (Pt), and gold (Au).44
Gold nanoparticles
Many efforts have been conducted on the development of Au-based nanosensors for environmental applications. This is due to the unique properties such as finely tunable optical properties, high surface area and high capacity for the surface modification. The Au nano-particles are considered as effective electrocatalyst in various electrochemical reactions because of their superior stability and complete recovery in chemical redox processes. The application of Au nanoparticles based electrodes has many advantages such as improved diffusion of electroactive species, high selectivity, improved catalytic activity and higher signal-to-noise ratio (S/N).45 Chen and co-workers,46 offered a simple and economical process for the fabrication of electrochemical gold –based nanosensors of the with low detection limit of 32.5 pM for arsenic ions (As3+).
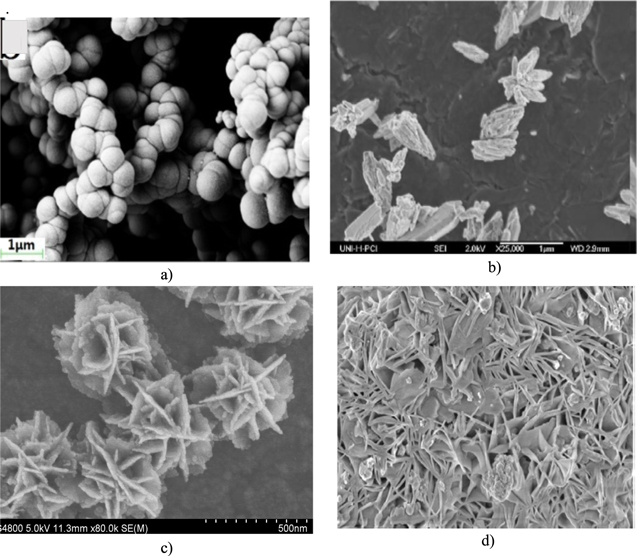
According to Ratner et al.,47 Au nanoparticles modified electrode surfaces showed a high sensitivity and sharper and more reproducible stripping peaks of mercury (Hg). Generally, the morphology of prepared nanoparticles can affect the sensitivity of nanosensors. Figure 3a, illustrates the morphology of Au dendrites that were developed by direct electrodeposition from a solution of HAuCl4 containing 3-aminopropyltriethoxysilane (APTS). The high performance of electrodeposited Au dendrites with high surface area was demonstrated in the enhanced sensitivity of glucose detection. This has potential applications in nonenzymatic electrochemical glucose biosensors.48
Figure 3. SEM images of noble metals with different morphologies used for nanosensors; (a) Au dendrites,49 (b) Silver dendritic nanostructures,58 (c) PtAu/rGO,61 (d) Pd/ZnO NW arrays.74
Download figure:
Standard image High-resolution imageGold nanoparticles are popularly used in biological and chemical sensors due to their fascinating chemical, optical, and catalytic properties.49 The inclusion of gold nanoparticles in modified electrodes facilitates the electron transfer between the transducer and biomolecules leading to better bioanalytical performance when redox enzymes and heme proteins are presented.50
Das et al.51 studied the behavior of gold nanoparticles as nanocatalysts for electrochemical protein detection.
According to the work conducted by Yuan et al.,52 Au nanoparticles are ultrasonically added to a carbonitride/graphene to form Au NPs/carbon nitride/graphene composite based electrochemical sensors. The interaction between Au NPs and GN/C3N4 allows improved charge transfer and provides effective catalytic effects and thus enhanced sensitivity. This material was used for sensitive detection of chloramphenicol and ciprofloxacin antibiotecs in food.
Silver nanoparticles
As a typical nanoparticle used in biosensors, the silver nanoparticles with controllable dimension and size distribution have attained much interest, due to its excellent surface-enhanced Raman scattering (SERS) and catalytic activity.53
According to Sebastian et al.,54 the Ag nanoparticles NP were prepared by microwave reactor. The Ag sensor exhibited a good limit of detection at 2.1 × 10−6 M of Hg (II) ions. The combination of the Ag nanoparticles with various matrixes such as metal oxides, silicate network, polymers, graphene, fibers, and dendrimers, provides high sensing efficiency with high stability because of the extended utility of the materials.
Kariuki and co-workers55 have synthesized an electrochemical Ag nanosensor based on Ag nanoparticles embedded in the poly (amic) acid (PAA) polymer matrix (PAA-Ag NPs) for detection of nitrobenzene. The PAA–Ag nano-particles based sensor showed a detection limit of 1.68 mM with a wide linear range of 10–600 mM and a high sensitivity of 7.88 mA mM−1 with low interference on structurally similar nitroaromatic compounds.
An electrochemical technique was recently developed by Sepunaru et al.56 for the efficient detection of influenza viruses tagged with the Ag nanoparticles. The current frequency and their magnitude increased linearly with increasing the concentration of virus as well as increasing the surface coverage of the nanoparticles. Silver nanoparticles based electrochemical immunosensors have the characteristics of a rapid response, high detectionsensitivity and specificity and easy fabrication. They have attracted much attention for monitoring numerous analytes, including small organic and inorganic molecules, microorganisms, and virus.
Many production techniques such as chemical reduction, layer-by-layer adsorption, template induction, photo-irradiation, seed-mediated synthesis, electroless preparation, and electrochemical deposition have been developed for the preparation of silver nanoparticles. Silver–DNA hybrid nanoparticles with controlled dimension were electrodeposited on a glassy carbon electrode by the reduction of silver with the aid of DNA.57
As electrode modification materials, dendritic structured materials with high specific surface area along, numerous active sites and sharp edges is critical for mass transfer and is in favor of heterogeneous catalysis. Dendritic silver nanostructures (Fig. 3b) were electrodeposited for H2O2 sensor application.58
The surface morphology of bare Si nanocolumns was modified by incorporating Ag NPs into Si nanocolumns by simple and fast immersions methods. A significant enhancement in sensitivity, response, and recovery times of gas sensor for a hybrid structure was realized after compared with Al/Si nanocolumns/n-Si/Al gas sensor due to the high specific surface area.59
Platinum nanoparticles
Platinum nanoparticles exhibit good catalytic properties and have been used in electrochemical analysis. For example, a highly sensitive H2O2 sensor based on the modification of a carbon film electrode with platinum nanoparticles has been developed by Lebegue et al.60 The modified electrode using platinum nanoparticles, exhibited sensitive response to H2O2 as compared to platinum bulk electrode. Safavi and Farjami61 prepared a biosensor by depositing gold–platinum (AuPt) alloy nanoparticles on glassy carbon electrode modified with an ionic liquid and chitosan composite, which was able to detect the reduction of H2O2 (Fig. 3c).
There are many fabrication techniques for platinum nanoparticles based electrode materials such as: chemical reduction, metal–vapor synthesis, electro-chemical and photochemical deposition. The selection of production technique plays an important role on developing the Pt nanoparticles on the numerous electrode surface, with superior chemical inertness, good stability, and low background current, high catalytic and sensing capabilities.62
The functionality of Pt nanomaterials is dependent on morphologically controlled interatomic bond distances, melting points, chemical reactivity, as well as optical and electronic properties. In addition, the chemical composition, surface condition, quality of crystal structure, crystallographic axis orientation, are important chracteristics of Pt nanomaterials that in return can affect electron transport mechanisms.62
Rismetov et al.63 have eletrodeposited pt nanoparticles on boron-doped diamond surface (BDD). The electrochemical deposition technique is suitable to construct the Pt nanoparticles on BDD electrode due to its simplicity and ease of fabrication. This Pt-based nanosensor was used for the detection of hydrogen peroxide (H2O2).
Using another production approach, Li et al.64 fabricated Pt-black coated Pt (Pt/Pt-black) based electrodes for detection of H2O2 and nitrite (NO2−) in PBS. The active surface area of the Pt/Pt-black electrodes allowed to avoid inhibition effect leading to long term stability compared to bare Pt electrodes. The presence of DNA is a key factor for the formation of homogenously distributed nano-sized nanoparticles with good catalytic ability. The Pt/Pt-black electrodes based sensor showed the detection limits of 10 and 12 nM for H2O2and NO2− respectively.
Zhang and coworkers65 have applied the hydrothermal process for the synthesizing of PtPd concave nanocubes dispersed in graphene nanoribbons (PtPd-rGO NRs) for the detection of trinitrotoluene (TNT). The PtPd-rGO NRs based nanosensor has demonstrated a wide linear range from 0.01 to 3 ppm with a detection limit of 0.8 ppb for TNT. Figure 3c demonstrates SEM image for PtAu/rGO nanomaterials.
Mahmoudian et al.66 reported the synthesis and characterization of polypyrrole coated nanospherical platinum (Pt/PPy NSs) for the detection of Hg2+. The electrochemical sensor showed a linear range between 5–500 nM with a detection limit of 0.27 nM for Hg2+. It exhibited a sensitivity of 1.239 mA nM−1 cm−2. The interference from other ions such as Ag+, Fe2+, Mn2+, K+, Pd2+, Cu2+, Ni2+, Pb2+, Sn2+ and Zn2+ was negligible, presenting an effective prospect for the detection of Hg2+.
Jung et al.67 have synthesized a potentiometric hydrogen sensor based on a Nafion electrolyte covered with Pt-C electrodes for the detection of hydrogen gas with high sensitivity, short response and recovery times, a wide linear range, and long-term stability.
Palladium nanoparticles
Palladium nanoparticles are characterized by extensive catalytic and sensor applications towards gases, biomolecules and hazardous toxic molecules. The Pd nanoparticle based electrode materials exhibit high electrocatalytic activities towards various analytes. The abundance of Pd over other noble metals such as Au and Pt, makes it a cheaper substitute for designing of various electrochemical sensors.68
The Pd based nanocomposites can improve the mass diffusion of the analytes. This in turn offered electron tunneling to enable the electron transfer between the active site and the electrode, leading to effective electrochemical sensing performance.68 He et al.69 demonstrated sensitive hydrazine sensor fabricated by one-step electrodeposition of palladium–graphene nanocomposites (Pd–GENCs) on indium tin oxide (ITO). The results of electrochemical tests indicated that under the optimum conditions, the current was dependent linearly on N2H4 concentrations in the range from 0.1 μM to 2.5 mM with a detection limit of 0.02 μM (S/N = 3) and sensitivity of 799.2 μA mM−1 cm−2. The response time of the sensor towards N2H4 was less than 3 s.
Due to their synergistic electronic effects, Pt nanoparticles dispersed on various substrates such as metal oxides, carbon nanotubes (CNTs), reduced graphene oxide (rGO), and rGO/ionic liquid (IL) composites, may enhance the oxidation of NO molecules in biomedical applications. A facile two-step electrodeposition process was developed in order to decorate the PGaN electrodes with Pd and Pt (Pd-Pt) nanocomposites. The sensor was characterized by low detection limit of 0.95 μM, superior linear ampere response and high sensitivity (150−A mM−1 for 1 to 300 −M and 73−A mM−1 for 300 to 3000−M), towards nitrite.62
Mahmoudian et al.70 have developed an electrochemical nitrate sensor based on polypyrrole PPy (Pd NCs-PPy) coated Pd nanoclusters. From the differential puls voltametry DPV results, the estimated limit of nitrates detection, limit of quantification (S/N = 3) for the two linear segments (lower and higher concentration of nitrate) were 0.7444, 2.4815 and 0.4535, 1.5117 μM, respectively.
An electrochemical H2O2 sensor based on the polyvinylpyrrolidone coated Pd nanoparticles (Pd NCs-PPy), was developed by Sophia et al.71 The vinyl polymers PVP offers the capability to keep the well-established catalytic activity of the metal nanoparticles intact with the chemical stability and the affinity of PVP towards the Pd metal.
According to research conducted by Tang and co-workers,72 palladium (Pd) nanoparticles were deposited on graphene using a new chemical method. Hydrogen sensors were fabricated using graphene decorated by Pd nanoparticles. The sensor showed a response of 5.88% for 1% H2 at room temperature under purple light illumination.
Lupan et al.73 stated that the presence of Pd nanoparticles on ZnO greatly enhances the room temperature catalytic activity due to the high H2 solubility in Pd. This gives higher concentration of clusters (catalytic centers) and lowers the saturation rate of response and recovery processes. A number of methods has been proposed to incorporate these metals into semiconducting oxides micro and nanostructures in order to improve their UV- and gas-sensing properties in the most efficient way. The most well-documented methods are adsorption, doping, surface functionalization (decorating, hybridization, loading, impregnating), and composing. Figure 3d demonstrates SEM image for Pd developed on ZnO nanorods.
According to Yi et al.,74 biosensors based on nano gold wires (NPG) decorated with palladium nanoparticles (Pd/NPG), showed tremendous superiority in the detection of DNA. This is due to the excellent catalytic activity of Pd and novel structure of NPG wires.
Copper
Copper has attracted many researchers as an ideal sensing material due to its good stability, excellent electricalconductivity, electrocatalytic properties and low cost compared with noble metal such as platinum, gold and silver. Copper nanostructures have many unique properties such as the high mass-transport rate, high surface to volume ratio, and the improved signal-to-noise ratio in electroanalytical measurements. Many synthetic methods such as reduction with hydrazine in ethylene glycol under microwave irradiation, the seed-mediated growth, the pulsed electrodeposition and deposition on some specific substrates are still complex and time-consuming.75
Li et al.75 prepared copper nano-clusters using a simple one-step electrodeposition process. The experimental results reveal that the porous copper layer electrodeposited under −700 mV is a high-performance electrocatalyst in facilitating nitrate reduction, and it also has a higher sensitivity of 39.31 μA mmol−1 L−1 for nitrate detection within the concentration ranging from 0.1 mmol L−1 to 4.0 mmol L−1.
Metal oxide nanoparticles
Metal oxide thin films and nanoparticles have the advantages of ultrahigh surface area, low cost, and unique properties. These ceramic-based nanomaterials have been widely used for fabricating nanosensors with high efficiency that can be used in different environmental and process monitoring, including combustion and emissions, petroleum refinery, and renewable energy technologies.76
Metal oxides (MOX) have a wide range of electronic, chemical, and physical properties that are often highly sensitive to changes in the chemical environment. Most commercial solid state chemical sensors are based on appropriately structured and doped metal oxides (mainly SnO2 and ZnO) that are capable of detecting a variety of gases with high sensitivity, good stability and also with low production cost. The fundamental sensing mechanism for the metal oxide-based gas sensors is based on the change in electrical conductivity due to charge transfer between surface complexes, such as O−, O2−, H+, and OH−, and interacting molecules. This process requires an activation energy so that classical MOX sensors are only functioning at high temperatures, generally above 200 °C.77
These metal oxides nanomaterials with large surface area, high adsorptive capacity, unique electrochemical activity and stability are of important for the design and synthesizing of electrochemical sensors. The analytical performance of the metal oxide nanomaterial based sensor is affected the morphology, particle size, surface area and surface functionality.78
The one dimensional (1D) nanostructures provide a great model system for electrochemical sensing of environmental pollutants. Resistive (conductometric) gas sensors based on nanostructured metal oxide semiconductors such as SnO2, In2O3, ZnO, TiO2, WO3 and NiO play an important role detection of environmental pollutants such as explosive/toxic gases and volatile organic compounds (VOCs). The operation principles of resistive gas sensor are based on the variation of resistance (electrical conductivity) caused by the change of test gas molecules on the electrodes surface. Many research activities have been conducted on the design and production of the hierarchical metal oxides nano-structures due to their smaller size and characteristic charge carriers, in order to improve the sensitivity and detection limit.79,80
Tin oxides
SnO2 nanoparticle is one of the most applied sensing materials for gas sensors. Khong et al.81 have investigated a hierarchical SnO2/ZnO nanostructure for high-performance ethanol sensors. As compared to the bare SnO2 NWs sensor, the hierarchical nanostructures higher sensitivity towards ethanol gas with better selectivity for interfering gases such as NH3, CO, H2, and CO2.
According to Pan.et al.,82 electrodeposited SnO2 with the addition of polyethylene glycol (PEG), as a surfactant. This surfactant led to the formation of spherical SnO2 nanoparticles (Fig. 4a). The obtained sensor showed better sensing performances for gases than the constructed from SnO2 prepared without the assistance of surfactant.
Figure 4. SEM of different metal oxides; (a) SnO2,82 (b) ZnO flakes,89 (c) ZnO nanoflowers,89 (d) NiO nanoflowers.91
Download figure:
Standard image High-resolution imageThe combination of SnO2 and reduced graphene oxide (rGO) was studied for the simultaneous and selective electrochemical detection of ultra-trace heavy metal ions in drinking water. The results were well satisfying the the World Health Organization (WHO).10
Zinc oxides
Due to their excellent electron transfer rate, ZnO nanostructures are able to evoke the hidden electrochemical ability of biomolecules, and facilitate their direct electrochemistry according to their excellent electron transfer rate.83 High surface to volume ratio, non-toxic, low cost, chemical stability, eco-friendly and high electron communication features than their bulk material are the main advantages of ZnO nanostructures.84 For instance, ZnO is a proper candidate for potential applications in gas sensing due to its thermal/chemical stability, good oxidation resistance, great bio-compatibility and high conductivity.85,86 ZnO is known as an n-type semiconductor having a wide band gap energy of 3.37 eV which can be used at high working temperatures of about 200 °C–450 °C.87
In the gas sensor, especially in ZnO-based sensors, the morphology of the sensing materials has an important role on their gas sensing properties.88 ZnO nanostructures have capability of low temperature growth with many different morphologies including wires, rods, tubes and flower shape (Figs. 4b, 4c).89 Flower-shape ZnO nanostructures have been synthesized using different methods of oxidation, reduction, decomposition and electrode position due to interesting structure, shape and properties. They have potential applications in electrochemical, electrical, optical and magnetic devices. These applications are due to low density, large active surface area, and surface permeability of these nanostructures.90
Generally, there are many methods for synthesizing ZnO nanostructures methods, such as vapor phase transport, magnetron sputtering, laser ablation, wet chemical methods including simple solution and hydrothermal and/or microwave treatment, depending upon their application. Microwave-assisted synthesis is considered as a simple and fast technique which has been used for many years for a variety of applications. The sensitivity and/or selectivity of the sensors such as optical, electronic and magnetic properties of ZnO can significantly be affected by additives.90
Akshaya Kumar et al.92applied the simple hydrothermal approach for the growth of ZnO nanorods (NRs) for the synthesizing of interdigitated electrodes (IDEs)-based pH sensor. The sensor showed sensitivity of 1.06 nF pH−1 in the range of pH 4−10. This type of sensors presents low-cost, and convenient device for measurement of pH in water.
The most common ammonia sensors are based on metal oxides such as ZnO, TiO2, CuO, SnO2, In2O3 and WO3. Schottky diodes based on AlGaN/GaN heterostructures (HEMTs) functionalized with ZnO nanorods were capable of ammonia detection in the range 0.1–2 ppm over the temperature range 25 °C–300 °C.92
Nickel oxides
NiO nanostructures are model semiconductors of p-type conductivity. They are used extensively in many applications, such as catalysis, battery electrodes, and gas sensors. The flower-like morphology of NiO (Fig. 4d)91 could enhance electrochemical activity of the electrode and provide larger contact area between active material and electrolyte. They have better electrochemical properties than conventional materials. The experimental results indicated the high sensitivity of rose-like NiO nanoparticles for formaldehyde gas sensing.10
Titanium oxides
TiO2 nanostructures also can be used on electrochemical sensors for medical and pharmaceutical applications. A range of proteins was immobilized into nanoporous TiO2 film electrodes. This technique was successfully used to develop electrochemical and optical biosensors. Li and co-workers93 investigated the performance of TiO2 nanostructures to entrap biomolecules such as cytochrome c, myoglobin, and hemoglobin, and studied the direct electrochemistry of these proteins.
Ardakani et al.94 modofied carbon paste electrodes by the addition TiO2 nanoparticles and meso-tetrakis (3-methylphenyl) cobalt porphyrin, used for the determination of levodopa in the presence of carbidopa. Differential pulse voltammetry DPV investigation technique showed the effective electrocatalytic activity of the modified electrodes in lowering the anodic overpotential for the oxidation of levodopa and complete resolution of its anodic wave from carbidopa.
Carbon based nanomaterials
Carbon based nano materials have excellent properties such as good conductivity, high stability, low cost, wide potential windows and easy surface functionalization.95 Carbon nanotubes (CNTs), graphene and nano/ mesoporous carbon were used for various electroanalytical applications. Their nanostructures provide efficient exposure of surface groups for the binding between analyte molecules and transduction material, leading to high detection performance for environmental pollutants.96,97
Carbon nanotubes
Carbon nanotubes (CNTs) are one of the most important materials because of their unique electronic, chemical, and mechanical properties since they were discovered by Sumio Iijima in 1991. CNTs is a 2D nanomaterial possessed sp2 carbon units with several nanometers in diameter and many microns in length. There are two types of CNTs, multi-walled (MW) and single-walled (SW). There are many production techniques for CNT such as electrical arc discharge, laser ablation, and chemical vapor deposition CVD methods. CNTs can be either the conductivity properties of metals or semiconductors, depending on the diameter and the degree of chirality. They have high electronic conductivity for the electron transfer reactions and better electrochemical and chemical stabilities in both aqueous and non-aqueous solutions.98
The sensing mechanism of CNT-based gas sensors is based on their p-type CNT semiconducting property. Generally, CNT electrical conductance is modified through the electron transfer between the CNTs and the oxidizing or reducing gas molecules adsorbed on the CNT surface. The electric resistance of p-type CNTs decreases with increasing the number of the adsorbed oxidizing gas molecules.99
Many CNTs based environmental nanosensors have been synthesized, including composite, pastes, film, and functionalised CNT sensors. This is due to the unique properties of large surface area, fast charge transfers as well as the compatibility and synergistic effect with the other electrode materials.
Maduraiveeran et al.100 have synthesized single-walled carbon nanotube nanosensors for the detection of the toxic phenolic compounds (catechol, p-cresol and p-nitrophenol), widely presented in aqueous and biological systems. This type of sensors exhibited high sensitivity, good reproducibility and stability.
Gooding et al.101 have discovered that the electroanalytical performance of SWNTs is more efficient than the corresponding MWNTs. For the SWNT based nanosensors, the oxygen-functionalized carbon nanotube is in direct contact with the solution, resulting in a fast electron transfer and excellent electrochemical detection. However, the reactions with MWNTs have been performed mainly with nanotubes in the non-oriented style and the sidewalls were mainly in contact with the solution, inhibiting the charge transport and thus reducing detection performance.
According to Shetti et al.,102 the inclusion of RuTiO2 nanoparticles and MWCNTs into the carbon matrix is launched as a best challenging composite material, and the tailored sensor stood proficient for the electrochemical study of clozapine drug 'CLZ'.
Zhao et al.103 developed MWNTs modified with polyamido sulfonic acid (PASA) film for the detection of hydroquinone and catechol. The response current using PASA/MWNTs/GC electrode was almost two times higher than the sum of peak currents at the PASA/GC and MWNTs/GC electrodes. This can be explained by the presence of the electron-rich N atoms and high SO3− electron density in the polymer film.
Carbon nanotubes (CNTs) can serve as scaffolds for immobilization of biomolecules at their surface, and combine several exceptional physical, chemical, electrical, and optical characteristics. This makes them one of the best materials for the transduction of signals associated with the recognition of analytes, metabolites, or disease biomarkers.104
Chen et al.105 reported aligned CNT biosensor as a uniform sensing platform that could be extended to real-time detections of various biomarkers.
Graphene
Graphene a unique two-dimensional nanostructure that allows fast electron transport. It has potential applications in the field of electrochemical sensors and biosensors.106 It has a theoretical surface area of 2630 m2 g−1, which is approximately 260 times greater than graphite and twice that of carbon nanotubes. Besides, it is a semiconductor with a zero band-gap, exhibiting ambipolar electric field effect with high charge carrier mobility (15,000–20,000 cm2/Vs). Graphene also possesses superior mechanical and thermal characteristics. Thus, graphene increases the electrochemical catalytic activity of the materials by greatly enlarging the surface area.107 There are many economic and high-yield processes for the production of graphene, such as the Hummers method rGO, electrochemical reduction, and chemical vapor deposition (CVD).108
The morphology and electrochemical properties of graphene makes it ideal for environmental sensing. Goh et al.109 have concluded the high performance of graphene nanoribbons-based electrodes toward electrochemical detection of explosive 2, 4, 6-trinitrotoluene (TNT).
Functionalization of graphene with various metal oxide nanoparticles can further improve the senstivity of graphene toward glucose detection. Metal oxide nanoparticles are excellent catalysts, due to their high ratio of surface atoms with free valences to the cluster of total atoms. They may even provide electrochemical reversibility for redox reactions.110,111
There are various techniques to deposit metal oxide nanostructures on graphene:
- 1.In situ chemical synthesis112
- 2.Hydrothermal processes113
- 3.Microwave heating technique114
- 4.Electrodeposition technique.
The growth mechanism of metal oxide MOX nanostructures on graphene is based on the attraction of positively-charged metal = metal-oxide ions by the polarized bonds of the functional groups on the graphene (such as –OH, C=O of carboxylic, O=C–O of carboxylate, C–O and O–C–O).115
Dai et al.116 have developed an electrochemical heavy metal ions sensor based on PPy/GO nanocomposites. This material was fabricated using in situ chemical oxidation polymerization and electrostatic functionalization. They exhibited high electrochemical conductivity and remarkable current increase as compared to PA/GO modified electrodes.
Luo et al.117 have developed graphene-cobalt hexacyanoferrate nanocomposites for the monitoring of carcinogenic hydrazine and nitrite. Graphene-based nanomaterials have been also successfully used for the detection of gaseous pollutants and heavy metal contaminants. Li et al.118 have fabricated high sensitivity electrodes made of channels of Pd-decorated rGO (Pd-RGO) and the chemical vapor deposition (CVD)-grown graphene. They were applied for nitric oxide (NO) gas detection.
Graphene nanocomposites have significant synergistic electrocatalytic effect toward the nitrite redox, which could improve the electrochemical response signals, and enhance the selectivity, sensitivity, and practicability for the nitrite detection in various environmental systems.119
It is worth mentioning that graphene is also playing an important role in the biosensor field due to its remarkable physical, optical, electrochemical and magnetic properties. Xu et al.120 discussed the commonly used prostate cancer (PC) protein biomarkers for biosensor, the unique properties of graphene and the roles of graphene-based materials for biosensing.
Porous carbon
Porous carbon is characterized by a high surface area, accessible surface chemistry, and short pathway for mass and electron transfer. It has attracted considerable attention in the field of electrochemical sensors. According to the International Union of Pure and Applied Chemistry (IUPA) classification, porous materials can be devided into three classifications based upon their pore sizes: microporous <2 nm, 2 nm <mesoporous <50 nm, and macroporous >50 nm.121
Ma et al.122 have developed on macro-/meso-porous carbon materials for the electrochemical detection of nitrobenzene (NB). They were fabricated by pyrolysis of the ionic liquid ([AEIm] BF4) polymer (PIL) pre-wrapped onto SiO2 microspheres and then removal of the silica core. The MMPCMs sensors showed stable, reproducible analytical performance for NB detection with a linear response range of 0.2–40 mM and the detection limit of 8 nM. This sensitive performance is due to the porous structure with large specific surface area and accumulation effect.
Niu et al.123 have synthesized a bismuth porous carbon nanocomposite based screen-printed electrodes (SPEs) for heavy metal detection. The nano-composite was synthesized via a combined one-step sol-gel and pyrolysis process, followed by the milling down to a specific particle size distribution for the screen printing ink. The resulting electrodes showed high sensitivity toward the detection of Pb2+ and Cd2+ ions at concentration levels below 4 ppb in tap drinking water and wastewater systems.
Wang et al.124 have integrated cobalt nanoparticles/3D-KSCs nanocomposite electrode with a 3D honeycomb porous structure. This nanostructure exhibited good electrocatalytic performances toward the oxidation and detection of amino acid.
Veerakumar and coworkers125 fabricated Pd nanoparticles (Pd NPs) dispersed on porous activated carbons (PACs) for the monitoring of toxic metal ions. The PACs are effectively employed as solid support for the dispersion of Pd nanoparticles. They have high porosities, high surface area and large pore volumes. They are suitable for the applications as nanosensors for detecting of multiple Cd2+, Pb2+, Cu2+, and Hg2+ metal ions with nano-molar detection limits.
Polymer and bio-nanomaterials
The nanostructures electrochemical sensors and biosensors based on polymeric and biomaterials showed high performance with rapid response and selectivity. This is attributed to their radiant, electrical, catalytic, mechanical, thermal and physical properties.126 Based on structural and functional complexity of polymeric and biomaterials, it is very difficult to determine the desired sensing properties. Using polymeric and bio-nanomaterials, the fabrication of electrochemical sensors can be achieved through the combination of novel analytical and scientific methods, including of combinatorial and high-throughput materials screening with micro- and nanofabrication and microfluidics.127
Polymer nanomaterials
Many effort on the technology of polymeric nanomaterials have been established for the detection of food and environmental pollutants.128 Polymeric nanomaterials provide many analytical strategies for the detection and determination of the chemically and biological toxic contaminations in gases and liquids for numerous health and environmental applications.129 The fabrication of the nano-composites with many combinations such as; metal nanoparticles, metal oxide nanoparticles, (carbon nanotube) CNT and graphene further improve the electrochemical sensing properties of polymeric nanomaterials.130,131
The combination of the matrix and nanofiller contributions are important in enhancing the biocompatibility, excellent sensitivity and selectivity. The polyaniline (PANI) nanofibers modified with bentonite nanohybrid were developed for gas sensor applications used for analysis of toxic gases such as acetone, benzene, ethanol and toluene.132 Navale et al.133 have investigated the gas sensing properties of polypyrrole (PPy)/a-Fe2O3 nanocomposites toward various oxidizing (NO2 and Cl2) and the reducing (CH3OH, C2H5OH, H2S and NH3) gases at room temperature. Recently, Lee et al.134 have fabricated poly(dopamine) (pDA)-modified indium tin oxide (ITO) electrodes for the detection of hydrazine (N2H4). In another work conducted by Liu et al.,135 single-walled carbon nanohorns (SWCNHs)–hollow Pt nanospheres/dendrimer sensors were prepared. The combination of high specific surface area of SWCNHs and the catalytically active Pt nano-particles were comprised in the biosensor.
Bio-nanomaterials
The combination of the catalytic function of biomolecules with special characteristics nanoscale materials provides numerous for nanosensors. A well-defined nanostructure with biomaterials can be obtained by the self-organization of biological molecules. Sabela and co-workers136 have developed MWNTs nanobiocomposite of L-phenylalanine ammonia-lyase enzyme for electrochemical biosensing of capsaicin. The developed biosensor showed a low detection limit of 0.18 mg mL−1. Li et al.137 have developed a self-assembled monolayers (SAMs) approach sensors for the detection of Escherichia coli O157:H7. This may lead to a portable biosensor method for routine monitoring of foodborne pathogens. The signal of the impedance can be altered by the immobilization of the biomaterials onto the surface of the printed interdigitated micro-electrodes (SPIMs). This developed immunosensor showed a low detection limit of 101 cfu ml−1 and a linear range from 102 to 107 cfu ml−1.
Conclusions
Many types of nanosensors has been reviewed, categorized and discussed according to energy source, structure, and materials. In general, optical nanosensors are very useful for chemicals monitoring inside a single cell. Electromagnetic nanosensors are applied for both chemical sensing as well as electromagnetic-mechatronic measurements. Meanwhile, mechanical nanosensors are used for determining the physico-mechanical properties and motion measurements. Many nanostructured materials applied for nanosensors were presented such as: metal, metal oxide, carbon nanotubes, graphene, polymers and biomaterials. Despite the relatively short history of nanosensors, the progress established in this area has been remarkable. With the continuing progress in nanotechnology tools and increasing research on the nano-scale phenomena, one may expect further achievements in the field of nanosensors. This can be reached through the enhanced performance of existing nanosensors and newer nanosensors based on novel mechanisms.