Abstract
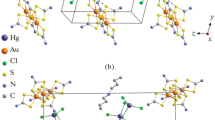
Chemisorption synthesis on the basis of the binuclear compound [Bi2{S2CN(C3H7)2}6] (I) and preparative isolation of the ion-polymeric heteronuclear gold(III)–bismuth(III) complex ([Au{S2CN(C3H7)2}2]3[Bi2Cl9])n (II) are carried out. Compounds I and II are characterized in comparison by IR spectroscopy and 13C CP-MAS NMR. According to the X-ray diffraction analysis data (CIF file CCDC no. 1407705), the cationic moiety of compound II exhibits an unusually complicated supramolecular structure including six isomeric noncentrosymmetric complex cations [Au{S2CN(C3H7)2}2]+ (hereinafter A–F) and two binuclear anions [Bi2Cl9]3– as conformers. The isomeric gold(III) cations perform various structural functions. Owing to pair secondary interactions Au···S, cations B, C, E, and F form centrosymmetric ([E···E], [F···F]) and noncentrosymmetric ([B···C]) binuclear aggregates [Au2{S2CN(C3H7)2}4]2+, whereas cations A and D are not involved in dimerization. The strongest secondary Au···S bonds are formed between the binuclear and mononuclear cations, resulting in the formation of supramolecular cation-cationic polymer chains of two types: (⋅⋅⋅A⋅⋅⋅[B⋅⋅⋅C]⋅⋅⋅A⋅⋅⋅[B⋅⋅⋅C]⋅⋅⋅)n and (D⋅⋅⋅[E⋅⋅⋅E]⋅⋅⋅D⋅⋅⋅[F⋅⋅⋅F]⋅⋅⋅])n. In both chains, the gold atoms of the binuclear cations are characterized by a distorted octahedral coordination [S6], whereas in the mononuclear cations the gold atoms retain the square environment [S4]. The cation-anionic interactions are provided by secondary bonds Cl⋅⋅⋅S involving the terminal chlorine atoms of isomeric [Bi2Cl9]3– and the sulfur atoms of the binuclear cations [Au2{S2CN(C3H7)2}4]2+. The character of the thermal behavior of compounds I and II is studied by simultaneous thermal analysis with the identification of intermediate and final products of the thermal transformations. The thermolysis of compound I at 193–320°C is accompanied by the formation of Bi2S3 with an impurity of reduced metallic bismuth particles. The final products of the thermal transformations of compound II are reduced elemental gold and Bi2O3, and the thermal transformation intermediates are BiCl3 and Bi2S3.







Similar content being viewed by others
Notes
The concept of secondary bonds was proposed [34] for the description of interactions characterized by the distances comparable with the sums of the van der Waals radii of the corresponding atoms.
The formation of metal sulfides upon the thermolysis of the complexes including the sulfur-containing ligands was substantiated from the thermodynamic point of view [42].
The evaporation of BiCl3 was studied for samples of the compact substance. A significant mass loss begins from sample melting, the evaporation region lies in a range of 234.0–415.0°С, and the maximum rate of mass loss falls on 376.6°С.
REFERENCES
de Carvalho, H.G. and de Araújo Penna, M., Lett. Nuovo Cimento, 1972, vol. 3, no. 18, p. 720.
de Marcillac, P., Coron, N., Dambier, G., et al., Nature, 2003, vol. 422, no. 6934, p. 876.
Li, H., Lai, C.S., Wu, J., et al., J. Inorg. Biochem., 2007, vol. 101, no. 5, p. 809.
Ishak, D.H.A., Ooi, K.K., Ang, K.-P., et al., J. Inorg. Biochem., 2014, vol. 130, p. 38.
Salvador, J.A.R., Figueiredo, S.A.C., Pinto, R.M.A., and Silvestre, S.M., Future Med. Chem., 2012, vol. 4, no. 11, p. 1495.
Guo, Y.-C., Ma, Q.-G., Chen, S.-Y., et al., Chin. J. Struct. Chem., 2015, vol. 34, no. 7, p. 1028.
Ozturk, I.I., Banti, C.N., Kourkoumelis, N., et al., Polyhedron, 2014, vol. 67, p. 89.
Arda, M., Ozturk, I.I., Banti, C.N., et al., RSC Adv., 2016, vol. 6, p. 29026.
Chauhan, H.P.S., Joshi, S., and Carpenter, J., J. Therm. Anal. Calorim., 2016, vol. 124, no. 1, p. 117.
Tamilvanan, S., Gurumoorthy, G., Thirumaran, S., and Ciattini, S., Polyhedron, 2017, vol. 121, p. 70.
Ariza-Roldán, A.O., López-Cardoso, E.M., Rosas-Valdez, M.E., et al., Polyhedron, 2017, vol. 134, p. 221.
Nomura, R., Kanaya, K., and Matsuda, H., Bull. Chem. Soc. Jpn., 1989, vol. 62, no. 3, p. 939.
Zhang, H., Huang, J., Zhou, X., and Zhong, X., Inorg. Chem., 2011, vol. 50, no. 16, p. 7729.
Kun, W.N., Mlowe, S., Nyamen, L.D., et al., Chem. Eur. J., 2016, vol. 22, no. 37, p. 13127.
Sivasekar, S., Ramalingam, K., Rizzoli, C., and Alexander, N., Inorg. Chim. Acta, 2014, vol. 419, p. 82.
Cabrita, J.F., Ferreira, V.C., and Monteiro, O.C., Electrochim. Acta, 2014, vol. 135, p. 121.
Abdullah, N.H., Zainal, Z., Silong, S., et al., Thermochim. Acta, 2016, vol. 632, p. 37.
Lai, C.S. and Tiekink, E.R.T., Z. Kristallogr., 2007, vol. 222, no. 10, p. 532.
Ivanov, A.V., Egorova, I.V., Ivanov, M.A., et al., Dokl. Phys. Chem., 2014, vol. 454, no. 1, p. 16.
Gowda, V., Sarma, B., Laitinen, R.S., et al., Polyhedron, 2017, vol. 129, p. 123.
Sun, R.-Z., Guo, Y.-C., Liu, W.-M., et al., Chin. J. Struct. Chem., 2012, vol. 31, no. 5, p. 655.
Zaeva, A.S., Ivanov, A.V., Gerasimenko, A.V., and Sergienko, V.I., Russ. J. Inorg. Chem., 2015, vol. 60, no. 2, p. 203. doi 10.1134/S0036023615020229
Zaeva, A.S., Ivanov, A.V., and Gerasimenko, A.V., Russ. J. Coord. Chem., 2015, vol. 41, no. 10, p. 644. doi 10.1134/S1070328415090109
Byr’ko, V.M., Ditiokarbamaty, Moscow: Nauka, 1984.
Pines, A., Gibby, M.G., and Waugh, J.S., J. Chem. Phys., 1972, vol. 56, no. 4, p. 1776.
APEX2, Madison: Bruker AXS Inc., 2010.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found Crystallogr., 2008, vol. 64, no. 1, p. 112.
Fabretti, A.C., Forghieri, F., Giusti, A., et al., Spectrochim. Acta, Part A, 1984, vol. 40, no. 4, p. 343.
Yin, H., Li, F., and Wang, D., J. Coord. Chem., 2007, vol. 60, no. 11, p. 1133.
Jaschinski, B., Blachnik, R., Pawlak, R., and Reuter, H., Z. Kristallogr. NCS, 1998, vol. 213, nos. 1−4, p. 541.
Savilov, S., Kloo, L., Kuznetsov, A., et al., Z. Anorg. Allg. Chem., 2003, vol. 629, no. 14, p. 2525.
Gerasimenko, A.V., Karaseva, E.T., and Polishchuk, A.V., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, vol. 64, no. 2, p. m378.
Winter, M., The Periodic Table of the Elements by Web Elements (accessed January 2010). http://www. webelements.com.
Alcock, N.W., Adv. Inorg. Chem. Radiochem., 1972, vol. 15, no. 1, p. 1.
Haiduc, I. and Edelmann, F.T., Supramolecular Organometallic Chemistry, Wiley-VCH, 1999.
Loseva, O.V. and Ivanov, A.V., Russ. J. Inorg. Chem., 2014, vol. 59, no. 12, p. 1491. doi 10.1134/ S0036023614120146
Ivanov, A.V., Loseva, O.V., Rodina, T.A., et al., Russ. J. Coord. Chem., 2016, vol. 42, no. 2, p. 104. doi 10.1134/S1070328416020032
Lalia-Kantouri, M., Christofides, A., and Manoussakis, G.E., J. Therm. Anal. Calorim., 1984, vol. 29, no. 2, p. 279.
Lalia-Kantouri, M. and Manoussakis, G.E., J. Therm. Anal. Calorim., 1984, vol. 29, no. 5, p. 1151.
Ripan, R. and Ceteanu, I., Neorganicheskaya khimiya. T. 1. Khimiya metallov, Spitsin, V.I., Kolli, I.D., Eds., Moscow: Mir, 1971.
Lidin, R.A., Andreeva, L.L., and Molochko, V.A., Konstanty neorganicheskikh veshchestv: spravochnik (Characteristics of Inorganic Compounds. A Handbook), Moscow: Drofa, 2008.
Razuvaev, G.A., Almazov, G.V., Domrachev, G.A., et al., Dokl. Akad. Nauk SSSR, 1987, vol. 294, no. 1, p. 141.
Larionov, S.V., Mikhalin, I.N., Glinskaya, L.A., et al., Russ. J. Inorg. Chem., 2004, vol. 49, no. 3, p. 331.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Ivanov, A.V., Gerasimenko, A.V., Egorova, I.V. et al. Chemisorption Synthesis of the Ion-Polymeric Heteronuclear Gold(III)–Bismuth(III) Complex ([Au{S2CN(C3H7)2}2]3[Bi2Cl9])n Based on [Bi2{S2CN(C3H7)2}6]: 13C MAS NMR, Supramolecular Structure, and Thermal Behavior. Russ J Coord Chem 44, 518–531 (2018). https://doi.org/10.1134/S1070328418080043
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328418080043