Abstract
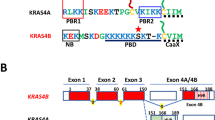
The C-terminal hypervariable domain of K-Ras4B targets the protein to the plasma membrane by a combination of positive charge and a hydrophobic signal (farnesyl group). We analysed the contribution of several structural features of the domain: net charge, charge distribution, amino acid sequence and lipid specificity to membrane targetting and function by using artificial ‘hypervariable’ domains fused to either EGFP or V12K-Ras4B. We found that charge and a lipid residue are sufficient for plasma membrane localization and function of the constitutively active V12K-Ras4B. However, the amount of net charge, charge distribution and the length of the anchoring domain are important. Increasing the net charge and concentrating it close to the C-terminus increases not only the percentage of membrane bound protein, but also shifts the distribution from internal membranes, including the nuclear envelope, to the plasma membrane. While plasma membrane binding is necessary for V12K-Ras4B activity (MAPK activation and focus formation), we found that there are additional restrictions. In particular, mutants with very highly charged domains that bind almost exclusively to the plasma membrane show less transforming potential than expected. In addition, a construct with a short ‘hypervariable’ domain (7 amino acids) also has decreased transformation activity. These results suggest that specific interactions between K-Ras4B and the plasma membrane are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ahuja HG, Foti A, Bar-Eli M and Cline MJ. . 1990 Blood 75: 1684–1690.
Barbacid M. . 1987 Annu. Rev. Biochem. 56: 779–827.
Booden MA, Baker TL, Solski PA, Der CJ, Punke SG and Buss JE. . 1999 J. Biol. Chem. 274: 1423–1431.
Bos JL. . 1989 Cancer Res. 49: 4682–4689.
Brandt-Rauf PW, Carty RP, Chem JM, Lee G, Rackovsky S and Pincus MR. . 1990 J. Protein Chem. 9: 137–142.
Buss JE, Solski PA, Schaeffer JP, MacDonald MJ and Der CJ. . 1989 Science 243: 1600–1603.
Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE and Philips MR. . 1999 Cell 98: 69–80.
Coats SG, Booden MA and Buss JE. . 1999 Biochemistry 38: 12926–12934.
Cox AD, Hisaka MM, Buss JE and Der CJ. . 1992 Mol. Cell. Biol. 12: 2606–2615.
Hagmann J, Grob M, Welman A, van Willigen G and Burger MM. . 1998 J. Cell. Sci. 111: 2181–2188.
Hagmann J, Burger MM and Dagan D. . 1999 J. Cell. Biochem. 73: 488–499.
Hancock JF, Cadwallader K, Paterson H and Marshall CJ. . 1991 EMBO J. 10: 4033–4039.
Hancock JF, Paterson H and Marshall CJ. . 1990 Cell 63: 133–139.
Hart KC and Donoghue DJ. . 1997 Oncogene 14: 945–953.
Herrmann C, Martin GA and Wittinghofer A. . 1995 J. Biol. Chem. 270: 2901–2905.
Jackson JH, Li JW, Buss JE, Der CJ and Cochrane CG. . 1994 Proc. Natl. Acad. Sci. USA 91: 12730–12734.
Johnson DR, Bhatnagar RS, Knoll LJ and Gordon JI. . 1994 Annu. Rev. Biochem. 63: 869–914.
Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, Bronson RT, Umanoff H, Edelmann W, Kucherlapati R and Jacks T. . 1997 Genes Dev. 11: 2468–2481.
Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, Van Aelst L, Wigler MH and Der CJ. . 1996 Mol. Cell. Biol. 16: 3923–3933.
Kikuchi A, Demo SD, Ye ZH, Chen YW and Williams LT. . 1994 Mol. Cell. Biol. 14: 7483–7491.
Koera K, Nakamura K, Nakao K, Miyoshi J, Toyoshima K, Hatta T, Otani H, Aiba A and Katsuki M. . 1997 Oncogene 15: 1151–1159.
Kuriyama M, Harada N, Kuroda S, Yamamoto T, Nakafuku M, Iwamatsu A, Yamamoto D, Prasad R, Croce C, Canaani E and Kaibuchi K. . 1996 J. Biol. Chem. 271: 607–610.
Laemmli UK. . 1970 Nature 227: 680–685.
Leventis R and Silvius JR. . 1998 Biochemistry 37: 7640–7648.
Lowy DR and Willumsen BM. . 1993 Annu. Rev. Biochem. 62: 851–891.
Ludin B, Doll T, Meili R, Kaech S and Matus A. . 1996 Gene 173: 107–111.
Malumbres M and Pellicer A. . 1998 Front Biosci. 6: 887–912.
McCabe JB and Berthiaume LG. . 1999 Mol. Biol. Cell. 10: 3771–3786.
Monaco R, Chen JM, Chung D, Brandt-Rauf P and Pincus MR. . 1995 J. Protein Chem. 14: 457–466.
Moodie SA, Willumsen BM, Weber MJ and Wolfman A. . 1993 Science 260: 1658–1661.
Niv H, Gutman O, Henis YI and Kloog Y. . 1999 J. Biol. Chem. 274: 1606–1613.
Okada Y and Hirokawa N. . 2000 Proc. Natl. Acad. Sci. USA 97: 640–645.
Qiu RG, Chen J, Kirn D, McCormick F and Symons M. . 1995 Nature 374: 457–459.
Resh MD. . 1996 Cell. Signal 8: 403–412.
Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD and Downward J. . 1994 Nature 370: 527–532.
Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD and Downward J. . 1996 EMBO J. 15: 2442–2451.
Roux P, Gauthier-Rouviere C, Doucet-Brutin S and Fort P. . 1997 Curr. Biol. 7: 629–637.
Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock JF and Parton RG. . 1999 Nat. Cell. Biol. 1: 98–105.
Siddiqui AA, Garland JR, Dalton MB and Sinensky M. . 1998 J. Biol. Chem. 273: 3712–3717.
Thissen JA, Gross JM, Subramanian K, Meyer T and Casey PJ. . 1997 J. Biol. Chem. 272: 30362–30370.
Voice JK, Klemke RL, Le A and Jackson JH. . 1999 J. Biol. Chem. 274: 17164–17170.
Vojtek AB, Hollenberg SM and Cooper JA. . 1993 Cell 74: 205–214.
Warne PH, Viciana PR and Downward J. . 1993 Nature 364: 352–355.
White MA, Vale T, Camonis JH, Schaefer E and Wigler MH. . 1996 J. Biol. Chem. 271: 16439–16442.
Willumsen BM, Cox AD, Solski PA, Der CJ and Buss JE. . 1996 Oncogene 13: 1901–1909.
Yan J, Roy S, Apolloni A, Lane A and Hancock JF. . 1998 J. Biol. Chem. 273: 24052–24056.
Acknowledgements
We thank Gema Alonso, Brian Hemmings and Andrew Matus for their helpful discussions. This work was supported by a grant to J Hagmann from the Swiss Cancer League (KFS 363-9-1996)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Welman, A., Burger, M. & Hagmann, J. Structure and function of the C-terminal hypervariable region of K-Ras4B in plasma membrane targetting and transformation. Oncogene 19, 4582–4591 (2000). https://doi.org/10.1038/sj.onc.1203818
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1203818
Keywords
This article is cited by
-
Remodeling of the Plasma Membrane by Surface-Bound Protein Monomers and Oligomers: The Critical Role of Intrinsically Disordered Regions
The Journal of Membrane Biology (2022)
-
Quantification of protein mobility and associated reshuffling of cytoplasm during chemical fixation
Scientific Reports (2018)
-
Prognostic significance of TRAIL death receptors in Middle Eastern colorectal carcinomas and their correlation to oncogenic KRAS alterations
Molecular Cancer (2010)