Abstract
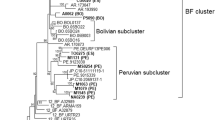
Two viruses, GB virus A (GBV-A) and GB virus B (GBV-B), were recently identified in the GB hepatitis agent. Human sera containing antibodies that recognize GBV-A and/or GBV-B recombinant proteins were subjected to polymerase chain reaction studies with degenerate oligonucleotides capable of amplifying a segment of the putative helicase genes from GBV-A, GBV-B or hepatitis C virus. Novel sequences related to members of the Flaviviridae were identified in sera from 12 individuals including 4 individuals with hepatitis. The limited nucleotide sequence identity between GBV-A, GBV-B and HCV sequences suggests that a novel virus, tentatively named GB virus C, may be responsible for some cases of non-A, non-B, non-C, non-D, non-E hepatitis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kuo, G. et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244, 362–364 (1989).
Dawson, G.J. et al. Solid-phase enzyme-linked immunosorbent assay for hepatitis E virus IgG and IgM antibodies utilizing recombinant antigens and synthetic peptides. J. Virol. Methods 38, 175–186 (1992).
Schlauder, G.G. & Mushahwar, I.K. Detection of hepatitis C and E virus by the polymerase chain reaction. J. Virol. Methods 47, 243–253 (1994).
Hollinger, F.B. et al. Transfusion-transmitted viruses study: Experimental evidence for two non-A, non-B hepatitis agents. J. infect. Dis. 142, 400–407 (1980).
Brotman, B., Prince, A.M. & Huima, T. Non-A, non-B hepatitis virus: Is there more than a single blood-borne strain? J. infect. Dis. 151, 618–625 (1985).
Tabor, E., April, M., Seef, L.B. & Gerety, R.J. Acquired immunity to human non-A, non-B hepatitis: Cross-challenge of chimpanzees with three infectious human sera. J. infect. Dis. 40, 789–793 (1979).
Yoshizawa, H. et al. Demonstration of two different types of non-A, non-B hepatitis by reinjection and cross-challenge studies in chimpanzees. Gastroenterology 81, 107–113 (1981).
Bradley, D.W. et al. Posttransfusion non-A, non-B hepatitis: Physiochemical properties of two distinct agents. J. infect. Dis. 148, 254–265 (1983).
Peters, T. et al. Frequency of hepatitis C in acute post-transfusion hepatitis after open-heart surgery: A prospective study in 1,476 patients. J. med. Virol. 39, 139–145 (1993).
Alter, H.J. Transfusion transmitted hepatitis C and non-A, non-B, non-C. Vox Sang. 67, 19–24 (1994).
Deinhardt, F., Holmes, A.W., Capps, R.B. & Popper, H. Studies on the transmission of disease of human viral hepatitis to marmoset monkeys. I. Transmission of disease, serial passage and description of liver lesions. J. exp. Med. 125, 673–687 (1967).
Holmes, A.W. et al. Specific neutralization of human hepatitis type A in marmoset monkeys. Nature 243, 419–420 (1973).
Deinhardt, F., Peterson, D., Cross, G., Wolfe, L. & Holmes, A.W. Hepatitis in marmosets. Am. J. med. Sci. 270, 73–80 (1975).
Tabor, E., Seeff, L.B. & Gerety, R.J. Lack of susceptibility of marmosets to human non-A, non-B hepatitis. J. infect. Dis. 140, 794–797 (1979).
Tabor, E., Peterson, D.A., April, M., Seeff, L.B. & Gerety, R.J. Transmission of human non-A, non-B hepatitis to chimpanzees following failure to transmit GB agent hepatitis. J. med. Virol. 5, 103–108 (1980).
Feinstone, S.M. et al. Non-A, non-B hepatitis in chimpanzees and marmosets. J. infect. Dis. 144, 588–598 (1981).
Whittington, R.O., Decker, R.H., Ling, C.-M. & Overby, L.R. in Viral and Immunological Diseases in Nonhuman Primates (ed. Kalter, S.S.) 221–224 (Liss, New York, 1983).
Karayiannis, P. et al. Studies of GB hepatitis agent in tamarins. Hepatology 9, 186–192 (1989).
Ticehurst, J. in Viral Hepatitis and Liver Disease (eds Hollinger, F.B., Lemon, S.M. & Margolis, H.S.) 501–513 (Williams & Wilkins, Baltimore, 1991).
Purcell, R.H. The discovery of the hepatitis viruses. Gastroenterology 104, 955–963 (1993).
Simons, J.N. et al. Identification of two flavivirus-like genomes in the GB hepatitis agent. Proc. natn. Acad. Sci. U.S.A. 92, 3401–3405 (1995).
Schlauder, G.G. et al. Molecular and serologic analysis in the transmission of the GB hepatitis agents. J. med. Virol. 46, 81–90 (1995).
Hibbs, J.R. et al. Aplastic anemia and viral hepatitis: non-A, non-B, non-C? JAMA 267, 2051–2054 (1992).
Sakamoto, M. et al. Entire nucleotide sequence and characterization of a hepatitis C virus of genotype V/3a. J. gen. Virol. 75, 1761–1768 (1994).
Stuyver, L. et al. Typing of hepatitis C virus isolates and characterization of new subtypes using a line probe assay. J. gen. Virol. 74, 1093–1102 (1993).
Sugitani, M., Inchauspé, G., Shindo, M. & Prince, A.M. Sensitivity of serological assays to identify blood donors with hepatitis C viraemia. Lancet 339, 1018–1019 (1992).
Simmonds, P. et al. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J. gen. Virol. 74, 2391–2399 (1993).
Bukh, J., Purcell, R.H. & Miller, R.H. At least 12 genotypes hepatitis C virus predicted by sequence analysis of the putative E1 gene of isolates collected worldwide. Proc. natn. Acad. Sci. U.S.A. 90, 8234–8238.
Iwarson, S. The natural course of chronic hepatitis C. FEMS Microbiol. Rev. 14, 201–204 (1994).
Cha, T.-A. et al. Use of a signature nucleotide sequence of hepatitis C virus for detection of viral RNA in human serum and plasma. J. clin. Microbiol. 29, 2528–2534 (1991).
Boiling, T.J. & Mandecki, W. An Escherichia coli expression vector for high-level production of heterologous proteins in fusion with CMP-KDO synthetase. Bio/Techniques 8, 488–490 (1990).
Paul, D.A. et al. Determination of hepatitis E virus seroprevalence by using recombinant fusion proteins and synthetic peptides. J. infect. Dis. 169, 801–806 (1994).
Roux, K.H. Using mismatched primer-template pairs in touchdown PCR. Bio/Techniques 16, 812–814 (1994).
Felsenstein, J. PHYLIP inference package, version 3. 5c (Distributed by the author, Dept. of Genetics, University of Washington, Seattle, 1993).
Choo, Q.-L. et al. Genetic organization and diversity of the hepatitis C virus. Proc. natn. Acad. Sci. U.S.A. 88, 2451–2455 (1991).
Okamoto, H. et al. Nucleotide sequences of the genomic RNA of hepatitis C virus isolated from a human carrier: Comparison with reported isolates for conserved and divergent regions. J. gen. Virol. 72, 2697–2704 (1991).
Okamoto, H. et al. Full-length sequence of a hepatitis C virus genome having poor homology to reported isolates: Comparative study of four distinct genotypes. Virology 188, 331–341 (1992).
Meyers, G., Ruemenapf, T. & Thiel, H.-J. Molecular cloning and nucleotide sequence of the genome of hog cholera virus. Virology 171, 555–567 (1989).
Collett, M.S. et al. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 165, 191–199 (1989).
Sumiyoshi, H. et al. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology 161, 497–510 (1987).
Dupuy, A., Despres, P., Cahour, A., Girard, M. & Bouloy, M. Nucleotide sequence comparison of the genome of two 17D-204 yellow fever vaccines. Nucleic Acids Res. 17, 3989–3989 (1989).
Castle, E., Leidner, U., Nowak, T. & Wengler, G. Primary structure of the West Nile flavivirus genome region coding for all nonstructural proteins. Virology 149, 10–26 (1986).
Fu, J., Tan, B.-H., Yap, E.-H., Chan, Y.-C. & Tan, Y.H. Full-length cDNA sequence of dengue type 1 virus (Singapore strain S275/90). Virology 188, 953–958 (1992).
Irie, K., Mohan, P.M., Sasaguri, Y., Putnak, R. & Padmanabhan, R. Sequence analysis of cloned dengue virus type 2 genome (New Guinea-C strain). Gene 75, 197–211 (1989).
Bukh, J., Purcell, R.H. & Miller, R.H. Importance of primer selection for the detection of hepatitis C virus RNA with the polymerase chain reaction assay. Proc. natn. Acad. Sci. USA. 89, 187–191 (1992).
Han, J.H. et al. Characterization of the terminal regions of hepatitis C viral RNA: Identification of conserved sequences in the 5′ untranslated region and poly(A) tails at the 3′ end. Proc. natn. Acad. Sci. U.S.A. 88, 1711–1715 (1991).
Koonin, E.V. & Dolja, V.V. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. mol. Biol. 28, 375–430 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Simons, J., Leary, T., Dawson, G. et al. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med 1, 564–569 (1995). https://doi.org/10.1038/nm0695-564
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm0695-564