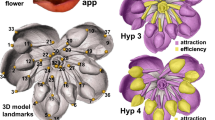
Abstract
Directional evolutionary trends have long garnered interest because they suggest that evolution can be predictable. However, the identification of the trends themselves and the underlying processes that may produce them have often been controversial1. In 1862, in explaining the exceptionally long nectar spur of Angraecum sesquipedale, Darwin proposed that a coevolutionary ‘race’ had driven the directional increase in length of a plant’s spur and its pollinator’s tongue2. Thus he predicted the existence of an exceptionally long-tongued moth. Though the discovery of Xanthopan morgani ssp. praedicta in 1903 with a tongue length of 22 cm validated Darwin’s prediction3, his ‘race’ model for the evolution of long-spurred flowers remains contentious4. Spurs may also evolve to exceptional lengths by way of pollinator shifts as plants adapt to a series of unrelated pollinators, each with a greater tongue length5. Here, using a species-level phylogeny of the columbine genus, Aquilegia, we show a significant evolutionary trend for increasing spur length during directional shifts to pollinators with longer tongues. In addition, we find evidence for ‘punctuated’ change in spur length during speciation events6, suggesting that Aquilegia nectar spurs rapidly evolve to fit adaptive peaks predefined by pollinator morphology. These findings show that evolution may proceed in predictable pathways without reversals and that change may be concentrated during speciation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gould, S. J. Cope’s rule as psychological artefact. Nature 385, 199–200 (1997)
Darwin, C. The Various Contrivances by which Orchids are Fertilized by Insects (John Murray, London, 1862)
Rothschild, L. W. & Jordon, K. A revision of the lepidopterous family Sphingidae. Novit. Zool. 9, 1–972 (1903)
Nilsson, N. Deep flowers for long tongues. Trends Ecol. Evol. 13, 259–260 (1998)
Wasserthal, L. T. The pollinators of the Malagasy star orchids Angraecum sesquipedale, A. sororium and A. compactum and the evolution of extremely long spurs by pollinator shift. Bot. Acta 110, 343–359 (1997)
Lee, C., Blay, S., Mooers, A., Singh, A. & Oakley, T. H. . CoMET: A Mesquite package for comparing models of continuous character evolution on phylogenies. Evol. Bioinform. Online 2, 193–196 (2006); 〈http://la-press.com/journals.php?pa=abstract&content_id=142〉.
Fulton, M. & Hodges, S. A. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proc. R. Soc. B 266, 2246–2252 (1999)
Johnson, S. D. & Steiner, K. E. Long-tongued fly pollination and evolution of floral spur length in the Disa draconis complex (Orchidaceae). Evolution Int. J. Org. Evolution 51, 45–53 (1997)
Wallace, A. R. Creation by law. Q. J. Sci. S140, 471–486 (1867)
Grant, V. & Temeles, E. J. Foraging ability of rufous hummingbirds on hummingbird flowers and hawkmoth flowers. Proc. Natl Acad. Sci. USA 89, 9400–9404 (1992)
Hodges, S. A. The influence of nectar production on hawkmoth behavior, self-pollination and seed production in Mirabilis multiflora (Nyctaginaceae). Am. J. Bot. 82, 197–229 (1995)
Grant, V. Pollination systems as isolating mechanisms in angiosperms. Evolution Int. J. Org. Evolution 3, 82–97 (1949)
Hodges, S. A. & Arnold, M. Columbines: A geographically widespread species flock. Proc. Natl Acad. Sci. USA 91, 5129–5132 (1994)
Hodges, S. A. & Arnold, M. Spurring plant diversification: Are floral nectar spurs a key evolutionary innovation? Proc. R. Soc. Lond. B 262, 343–348 (1995)
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17, 754–755 (2001)
Grant, V. Isolation and hybridization between Aquilegia formosa and A. pubescens. Aliso 2, 341–360 (1952)
Hodges, S. A., Fulton, M., Yang, J. Y. & Whittall, J. B. Verne Grant and evolutionary studies of Aquilegia. New Phytol. 161, 113–120 (2004)
Pagel, M., Meade, A. & Barker, D. Bayesian estimation of ancestral character states on phylogenies. Syst. Biol. 53, 673–684 (2004)
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985)
Butler, M. A. & King, A. A. Phylogenetic comparative analysis: A modelling approach for adaptive evolution. Am. Nat. 164, 683–695 (2004)
Miller, W. E. Diversity and evolution of tongue length in hawkmoths (Sphingidae). J. Lepid. Soc 5, 9–31 (1997)
Hundsdoerfer, A. K., Kitching, I. J. & Wink, M. A molecular phylogeny of the hawkmoth genus Hyles (Lepidoptera: Sphingidae, Macroglossinae). Mol. Phylogenet. Evol. 35, 442–458 (2005)
Kay, K., Whittall, J. B. & Hodges, S. A. A survey of nrITS substitution rates across angiosperms: An approximate molecular clock with life history effects. BMC Evol. Biol. 6, 36 (2006)
Vos, P. et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 23, 4407–4414 (1995)
Sanderson, M. J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003)
Pagel, M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Syst. Biol. 48, 612–622 (1999)
Midford, P., Garland, T. Jr. & Maddison, W. P. PDAP package of Mesquite. Version 1.07 (2005); 〈http://mesquiteproject.org/pdap_mesquite/index.html〉.
Maddison, W. P. & Maddison, D. R. . Mesquite: A modular system for evolutionary analysis. Version 1.05 (2004); 〈http://mesquiteproject.org/mesquite/mesquite.html〉.
Garland, T., Harvey, P. H. & Ives, A. R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 41, 18–32 (1992)
Acknowledgements
We thank D. Bush and B. Counterman for laboratory assistance; J. Yang, M. Fulton, S. Schaefer, Y. Kisel and J. Bean for greenhouse and field help; T. Oakley and B. O’Meara for technical assistance; C. Voelkel and C. Eckert for comments on earlier versions of this manuscript; and M. Hodges for help with the figures. Funding was provided by the Botanical Society of America, UCSB’s Olivia Long Converse Fellowship, the National Science Foundation, UC Davis’ Comparative Biology Postdoctoral Fellowship, and the Native Plant Societies of Wyoming, Colorado, and northern Nevada.
Author Contributions J.B.W. and S.A.H. designed the study and collected the samples; J.B.W. collected and analysed the phylogenetic and comparative data; J.B.W. and S.A.H. wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Discussion, Supplementary Figure 1 with Legend, Supplementary Tables 1-5 and additional references. (PDF 481 kb)
Rights and permissions
About this article
Cite this article
Whittall, J., Hodges, S. Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447, 706–709 (2007). https://doi.org/10.1038/nature05857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05857
This article is cited by
-
Genetic basis of nectar guide trichome variation between bumblebee- and self-pollinated monkeyflowers (Mimulus): role of the MIXTA-like gene GUIDELESS
BMC Plant Biology (2024)
-
Lack of pollinators selects for increased selfing, restricted gene flow and resource allocation in the rare Mediterranean sage Salvia brachyodon
Scientific Reports (2024)
-
The scaling relationship between perianth fresh mass and area: proof of concept using Magnolia × soulangeana Soul.-Bod
Trees (2024)
-
Floral nectaries and pseudonectaries in Eranthis (Ranunculaceae): petal development, micromorphology, structure and ultrastructure
Protoplasma (2022)
-
Optimization of SPME–GC–MS and characterization of floral scents from Aquilegia japonica and A. amurensis flowers
BMC Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.