Abstract
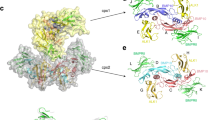
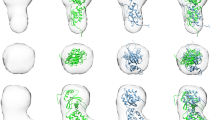
Bone morphogenetic proteins (BMPs) belong to the large transforming growth factor-β (TGF-β) superfamily of multifunctional cytokines. BMP-2 can induce ectopic bone and cartilage formation in adult vertebrates and is involved in central steps in early embryonal development in animals. Signaling by these cytokines requires binding of two types of transmembrane serine/threonine receptor kinase chains classified as type I and type II. Here we report the crystal structure of human dimeric BMP-2 in complex with two high affinity BMP receptor IA extracellular domains (BRIAec). The receptor chains bind to the ‘wrist’ epitopes of the BMP-2 dimer and contact both BMP-2 monomers. No contacts exist between the receptor domains. The model reveals the structural basis for discrimination between type I and type II receptors and the variability of receptor–ligand interactions that is seen in BMP–TGF-β systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Massagué, J. Annu. Rev. Biochem. 67, 753–791 (1998).
Liu, F., Ventura, F., Doody, J. & Massague, J. Mol. Cell. Biol. 15, 3479–3486 (1995).
ten Dijke, P. et al. J. Biol. Chem. 269, 16985–16988 (1994).
Mittl, P.R. et al. Protein Sci. 5, 1261–1271 (1996).
Scheufler, C., Sebald, W. & Hülsmeyer, M. J. Mol. Biol. 287, 103–115 (1999).
Hinck, A.P. et al. Biochemistry 35, 8517–8534 (1996).
Griffith, D.L., Keck, P.C., Sampath, T.K., Rueger, D.C. & Carlson, W.D. Proc. Natl. Acad. Sci. USA 93, 878–883 (1996).
Daopin, S., Piez, K.A., Ogawa, Y. & Davies, D.R. Science 257, 369–373 (1992).
Schlunegger, M.P. & Grutter, M.G. Nature 358, 430–434 (1992).
Eigenbrot, C. & Gerber, N. Nature Struct. Biol. 4, 435–438 (1997).
Greenwald, J., Fischer, W.H., Vale, W.W. & Choe, S. Nature Struct. Biol. 6, 18–22 (1999).
Rees, B. & Bilwes, A. Chem. Res. Toxicol. 6, 385–406 (1993).
Isaacs, N.W. Curr. Opin. Struct. Biol. 5, 391–395 (1995).
Wells, J.A. et al. Recent Prog. Horm. Res. 48, 253–275 (1993).
Hage, T., Sebald, W. & Reinemer, P. Cell 97, 271–281 (1999).
Huang, S.S., Zhou, M., Johnson, F.E., Shieh, H.S. & Huang, J.S. J. Biol. Chem. 274, 27754–27758 (1999).
Gray, P.C. et al. J. Biol. Chem. 275, 3206–3212 (2000).
Lux, A., Attisano, L. & Marchuk, D.A. J. Biol. Chem. 274, 9984–9992 (1999).
Wiesmann, C. et al. Cell 91, 695–704 (1997).
Wiesmann, C., Ultsch, M.H., Bass, S.H. & de Vos, A.M. Nature 401, 184–188 (1999).
Ruppert, R., Hoffmann, E. & Sebald, W. Eur. J. Biochem. 237, 295–302 (1996).
Kirsch, T., Nickel, J. & Sebald, W. FEBS Lett. 468, 215–219 (2000).
Kabsch, W. J. Appl. Crystallogr. 26, 795–800 (1993).
Collaborative Computational Project, Number 4. CCP4 Suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994).
Brunger, A.T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Carson, M. J. Applied Crystallogr. 24, 958–961 (1991).
Nicholls, A., Sharp, K.A. & Honig, B. Proteins 11, 281–296 (1991).
Corpet, F. Nucleic Acids Res. 16, 10881–10890 (1988).
Acknowledgements
We thank P. Knaus for critical reading of the manuscript and M. Gottermeier for technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 487).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirsch, T., Sebald, W. & Dreyer, M. Crystal structure of the BMP-2–BRIA ectodomain complex. Nat Struct Mol Biol 7, 492–496 (2000). https://doi.org/10.1038/75903
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/75903
This article is cited by
-
Rational Identification of Conformational and Linear EGFR Epitopes Recognized Specifically by, Respectively, Type-I and Type-II Anti-EGFR Antibodies and Molecular Design of Linear Epitope-Derived Peptidic Mimotopes to Elicit Type-II Antibody
International Journal of Peptide Research and Therapeutics (2023)
-
The versatility and paradox of BMP signaling in endothelial cell behaviors and blood vessel function
Cellular and Molecular Life Sciences (2022)
-
Endometrial receptivity and implantation require uterine BMP signaling through an ACVR2A-SMAD1/SMAD5 axis
Nature Communications (2021)
-
Expression of BMP2-Hydrophobin fusion protein in the tobacco plant and molecular dynamic evaluation of its simulated model
Plant Biotechnology Reports (2021)
-
Familial juvenile polyposis syndrome with a de novo germline missense variant in BMPR1A gene: a case report
BMC Medical Genetics (2020)