Abstract
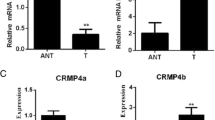
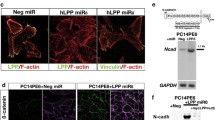
Numerous genetic changes are associated with metastasis of cancer cells. Previously, we used microarray to identify that collapsin response mediator protein-1 (CRMP-1) was involved in cancer invasion and metastasis. We further characterized that CRMP-1 was a novel invasion-suppression gene. Members of the CRMP gene family are intracellular phosphoproteins involved in the mediation of semaphorin induced F-actin depolymerization and growth cone collapse. The precise mechanism by which CRMP-1 inhibits invasion is not yet clear. However, CRMP-1 transfected cells had fewer filopodia and less Matrigel-invasion abilities. A low expression of CRMP-1 mRNA in lung cancer tissue was significantly associated with advanced disease, lymph node metastasis, early post-operative relapse, and shorter survival. In this article, we reviewed the functions of CRMPs and semaphorins and analyzed the structure and motifs of CRMP-1 by bioinformatics. As such, we hoped to shed further light on the mechanism by which CRMP-1 suppresses the invasion of cancer cells.
Similar content being viewed by others
References
Liotta LA, Rao CN, Wewer UM. Biochemical interactions of tumor cells with the basement membrane. Ann Rev Biochem 1986; 55: 1037–57.
Yoshida BA, Sololoff MM, Welch DR et al. Metastasis-suppressor genes: A review and perspective. J Natl Cancer Inst 2000; 92 (21): 1717–30.
Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer 1997; 80(8 Suppl): 1529–37.
Fildler IJ, Kripke ML.Metastasis results from preexisting variant cells within a malignant tumor. Science 1977; 197(4306): 893–5.
Chu YW, Yang PC, Yang SC et al. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am J Respir Cell Mol Biol 1997; 17(3): 353–60.
Chen JJW, Peck K, Hong TM et al. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res 2001; 61(13): 5223–30.
Ross DT, Scherf U, Eisen MB et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 2000; 24(3): 227–35.
Ramaswarmy S, Golub TR. DNA microarray in clinical oncology. J Clin Oncol 2002; 20(7): 1932–41.
Chen JW, Wu R, Yang PC et al. Profiling expression patterns and isolating differentially expressed genes by cDNA microarray system with colorimetric detection. Genomics 1998; 51(3): 313–24.
Hong TM, Yang PC, Peck K et al. Profiling the downstream genes of tumor suppressor PTEN in lung cancer cells by complementary DNA microarray. Am J Respir Cell Mol Biol 2000; 23(3): 355–63.
Shih JY, Yang SC, Hong TM et al. Collapsin response mediator protein-1 and the invasion and metastasis of cancer cells. J Natl Cancer Inst 2001; 93(18): 1392–400.
Quinn CC, Gray GE, Hockfield SA. Family of proteins implicated in axon guidance and outgrowth. J Neurobiol 1999; 41(1): 158–64.
Fukada M, Watakabe I, Yuasa-Kawada J et al. Molecular characterization of CRMP5, a novel member of the collapsin response mediator protein family. J Biol Chem 2000; 275(48): 37957–65.
Gaetano C, Matsuo T, Thiele CJ. Identification and characterization of a retinoic acid-regulated human homologue of the unc-33-like phosphoprotein (hUlip) from neuroblastoma cells. J Biol Chem 1997; 272(18): 12195–201.
Goshima Y, Nakamura F, Strittmatter P et al. Collapsin-induced growth cone collapse mediated by an intracellular protein related to unc-33. Nature 1995; 376(6540): 509–14.
Wang LH, Stittmatter SM. A family of rat CRMP genes is differentially expressed in the nervous system. J Neruosci 1996; 16(19): 6197–207.
Hamajima N, Matsuda K, Sakata S et al. A novel gene family de-fined by human dihydropyrimidinase and three related proteins with differential tissue distribution. Gene 1996; 180(1-2): 157–63.
Byk T, Dobransky T, Cifuentes-Diaz C et al. Identification and molecular characterization of unc-33-like phosphoprotein (Ulip), a putative mammalian homology of the axonal guidance-associated unc-33 gene product. J Neurosci 1996; 16(2): 688–701.
Minturn JE, Fryer HJ, Geschwind DH et al. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J Neurosci 1995; 15(10): 6757–66.
Li W, Herman RK, Shaw JE. Analysis of the Caenorhabditis elegans axonal guidance and outgrowth gene unc-33. Genetics 1992; 132(3): 675–89.
Arimura N, Inagaki N, Chihara K et al. Phosphorylation of collapsin response mediator protein-2 by rho-kinase. J Biol Chem 2000; 275(31): 23973–80.
Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science 1996; 274(5290): 1123–33.
He Z, Wang KC, Koprivica V et al. Knowing how to navigate: mechanisms of semaphorin signaling in the nervous system. Sci STKE 2002; 2002(119): RE1.
Goshima Y, Ito T, Sasaki Y et al. Semaphorins as signals for cell repulsion and invasion. J Clin Invest 2002; 109(8): 993–8.
Bismuth G, Boumsell L. Controlling the immune system through semaphorins. Sci STKE 2002; 2002(128): RE4.
Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol 2000; 10(1): 88–94.
Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993; 75(2): 217–27.
EicKholt B, Mackenzie SL, Graham A et al. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrian regions. Development 1999; 126(10): 2181–9.
Fan J, Mansfield SG, Redmond T et al. The organization of Factin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol 1993; 121(4): 867–78.
Miao HQ, Soker S, Feiner L et al. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol 1999; 146(1): 233–42.
Cooper JA. The role of actin polymerization in cell motility. Annu Rev Physiol 1991; 53: 585–605.
Aizawa H, Wakatsuki S, Ishii A et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 2001; 4(4): 367–73.
Neufeld G, Cohen T, Shraga N et al. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc Med 2002; 12(1): 13–9.
Mamluk R, Gechtman Z, Kutcher ME et al. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2 and heparin via Its b1b2 domain. J Biol Chem 2002 (in press).
Miao HQ, Lee P, Lin H et al. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J 2000; 14(15): 2532–9.
Zochbauer-Muller S, Gazdar AF, Minna JD. Molecular pathogenesis of lung cancer. Annu Rev Physiol 2002; 64: 681–708.
Roche J, Boldog F, Robinson M et al. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene 1996; 12(6): 1289–97.
Sekido Y, Bader S, Latif F et al. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demon strate distinct expression patterns. Proc Natl Acad Sci USA 1996; 93(9): 4120–5.
Xiang RH, Hensel CH, Garcia DK et al. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics 1996; 32(1): 39–48.
Gutgemann A, Golob M, Muller S et al. Isolation of invasionassociated cDNAs in melanoma. Arch Dermatol Res 2001; 293(6): 283–90.
Tomizawa Y, Sekido Y, Kondo M et al. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci USA. 2001; 98(24): 13954–9.
Tse C, Xiang RH, Bracht T et al. Human Semaphorin 3B (SEMA3B) located at chromosome 3p21.3 suppresses tumor formation in an adenocarcinoma cell line. Cancer Res 2002; 62(2): 542–6.
Brambilla E, Constantin B, Drabkin H et al. Semaphorin SEMA3F localization in malignant human lung and cell lines: A suggested role in cell adhesion and cell migration. Am J Pathol 2000; 156(3): 939–50.
Xiang R, Davalos AR, Hensel CH et al. Semaphorin 3F gene from human 3p21.3 suppresses tumor formation in nude mice. Cancer Res 2002; 62(9): 2637–43.
Comoglio PM, Trusolino L. Invasive growth: From development to metastasis. J Clin Invest 2002; 109(7): 857–62.
Yamada T, Endo R, Gotoh M et al. Identification of semaphorin E as s non-MDR drug resistance gene of human cancers. Proc Natl Acad Sci USA 1997; 94(26): 14713–8.
Martin-Satue M, Blanco J. Identification of semaphorin E gene expression in metastatic human lung adenocarcinoma cells by mRNA differential display. J Surg Oncol 1999; 72(1): 18–23.
Christensen CR, Klingelhofer J, Tarabykina S et al. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res 1998; 58(6): 1238–44.
Holm L, Sander C. An evolutionary treasure: unification of a broad set of amidohydrolases related to urease. Proteins 1999; 28(1): 72–82.
Thoden JB, Phillips GN Jr, Neal TM et al. Molecular structure of dihydroorotase: a paradigm for catalysis through the use of a binuclear metal center. Biochemistry 2001; 40(24): 6989–97.
Wang LH, Strittmatter SM. Brain CRMP forms heterotetramers similar to liver dihydropyrimidinase. J Neurochem 1997; 69(6): 2261–9.
Kikugawa M, Kaneko M, Fujimoto-Sakata S et al. Purification, characterization and inhibition of dihydropyrimidinase from rat liver. Eur J Biochem 1994; 219(1-2): 393–9.
Neer EJ, Schmidt CJ, Nambudripad R et al. The ancient regulatoryprotein family of WD-repeat proteins. Nature 1994; 371(6495): 297–300.
Smith J. Brachyury and the T-box genes. Curr Opin Genet Dev 1997; 7(4): 474–80.
Smith J. T-box genes: What they do and how they do it. Trends Genet 1999; 15(4): 154–8.
Wattler S, Russ A, Evans M et al. A combined analysis of genomic and primary protein structure defines the phylogenetic relationship of new members if the T-box family. Genomics 1998; 48(1): 24–33.
Kavka AI, Green JB. Tales of tails: Brachyury and the T-box genes. Biochim Biophys Acta 1997; 1333(2): F73–84.
Papaioannou VE. T-box family reunion. Trends Genet 1997; 13(6): 212–3.
Muller CW, Herrmann BG. Crystallographic structure of the T domain-DNA complex of the Brachyury transcription factor. Nature 1997; 389(6653): 884–8.
Klotzbucher A, Stewart E, Harrison D et al. The ‘destruction box’ of cyclin A allows B-type cyclins to be ubiquitinated, but not efficiently destroyed. EMBO J 1996; 15(12): 3053–64.
Yamano H, Tsurumi C, Gannon J et al. The role of the destruction box and its neighbouring lysine residues in cyclin B for anaphase ubiquitin-dependent proteolysis in fission yeast: defining the D-box receptor. EMBO J 1998; 17(19): 5670–8.
Whitfield ML, Sherlock G, Saldanha A et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 2002 (in press).
Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics 1992; 14: 897–911.
Einbond A, Sudol M. Towards prediction of cognate complexes between the WW domain and proline-rich ligands. FEBS Lett 1996; 384(1): 1–8.
Freund C, Dotsch V, Nishizawa K et al. The GYF domain is a novel structural fold that is involved in lymphoid signaling through prolinerich sequences. Nat Struct Biol 1999; 6(7): 656–60.
Ball LJ, Jarchau T, Oschkinat H et al. EVH1 domains: Structure, function and interactions. FEBS Lett 2002; 513(1): 45–52.
Ko L, Cardona GR, Chin WW. Thyroid hormone receptor-binding protein, an LXXLL motif-containing protein, functions as a general coactivator. Proc Natl Acad Sci USA 2000; 97(11): 6212–7.
Inagaki H, Kato Y, Hamajima N et al. Differential expression of dihydropyrimidinase-related protein genes in developing and adult enteric nervous system. Histochem Cell Biol 2000; 113(1): 37–41.
Matsuo T, Stauffer JK, Walker RL et al. Structure and promoter analysis of the human unc-33-like phosphoprotein gene: E-box required for maximal expression in neuroblastoma and myoblasts. J Biol Chem 2000; 275(22): 16560–8.
Quach TT, Mosinger B Jr, Ricard D et al. Collapsin response mediator protein-3/unc-33-like protein-4 gene: organization, chromosomal mapping and expression in the developing mouse brain. Gene 2000; 242(1-2): 175–82.
Byk T, Ozon S, Sobel A. The Ulip family phosphoproteins: Common and specific properties. Eur J Biochem 1998; 254(1): 14–24.
Inagaki N, Chihara K, Arimura N et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci 2001; 4(8): 781–2.
Gu Y et al. Neurofibrillary tangle-associated collapsin response mediator protein-2 (CRMP-2) is highly phosphorylated on Thr-509, Ser-518, and Ser-522. Biochemistry 2000, 39(15): 4267–75.
Cole RN, Hart GW. Cytosolic O-glycosylation is abundant in nerve terminals. J Neurochem 2001; 79(5): 1080–9.
Gu Y, Ihara YJ. Evidence that collapsin response mediator protein-2 is involved in the dynamics of microtubules. J Biol Chem 2000; 275(24): 17917–20.
Mack GJ, Ou Y, Rattner JB. Integrating centrosome structure with protein composition and function in animal cells. Microsc Res Tech 2000; 49(5): 409–19.
Tassin AM, Bornens M. Centrosome structure and microtubule nucleation in animal cells. Biol Cell 1999; 91(4-5): 343–54.
Morris VB, Brammall J, Noble J et al. p53 localizes to the centrosomes and spindles of mitotic cells in the embryonic chick epiblast, human cell lines, and a human primary culture: an immunofluorescence study. Exp Cell Res 2000; 256(1): 122–30.
Hsu LC, White RL. BRCA1 is associated with the centrosome during mitosis. Proc Natl Acad Sci USA 1998; 95(22): 12983–8.
Nishiyama Y, Hirota T, Morisaki T et al. A human homolog of Drosophila warts tumor suppressor, h-warts, localized to mitotic apparatus and specifically phosphorylated during mitosis. FEBS Lett 1999; 459(2): 159–65.
Honnorat J, Byk T, Kusters I et al. Ulip/CRMP proteins are recognized by autoantibodies in paraneoplastic neurological syndromes. Eur J Neurosci 1999; 11(12): 4226–32.
Yu Z, Kryzer TJ, Griesmann GE et al. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 2001; 49(2): 146–54.
Antoine JC, Honnorat J, Camdessanche JP et al. Paraneoplastic anti-CV2 antibodies react with peripheral nerve and are associated with a mixed axonal and demyelinating peripheral neuropathy. Ann Neurol 2001; 49(2): 214–21.
Bulliard C, Zurbriggen R, Tornare J et al. Purification of a dichlorophenol-indophenol oxidoreductase from rat and bovine synaptic membranes: tight assocation of a glycraldehyde-3-phosphate dehydrognease isoform, TOAD-64, enolase-γ and aldolase C. Biochem J 1997; 324 (Pt 2): 555–63.
Lee S, Kim JH, Lee CS et al. Collapsin response mediator protein-2 inhibits neuronal phospholipase D(2) activity by direct interaction. J Biol Chem 2002; 277(8): 6542–9.
Li B, Perabekam S, Liu G et al. Experimental and bioinformatics comparison of gene expression between T cells from TIL of liver cancer and T cells from UniGene. J Gastroenterol 2002; 37(4): 275–82.
Rouzaut A, Lopex-Moratalla N, de Miguel C. Differential gene expression in the activation and maturation of human monocytes. Arch Biochem Biophys 2000; 374(2): 153–60.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shih, JY., Lee, YC.G., Yang, SC. et al. Collapsin response mediator protein-1: A novel invasion-suppressor gene. Clin Exp Metastasis 20, 69–76 (2003). https://doi.org/10.1023/A:1022598604565
Issue Date:
DOI: https://doi.org/10.1023/A:1022598604565