Abstract
A lake bank filtration (LBF) scheme comprising of nine tubewells on the bank of the lake Naini in Nainital, India exists in landslide debris while most bank filtration sites globally are in alluvial aquifers. The water quality and stable isotopes (δ18O and δ2H) have been studied to assess the proportions of bank filtrate drawn by the wells. Results show that of the nine wells, two wells perennially abstract mainly bank filtrate, three abstract predominantly bank filtrate during non-monsoon but groundwater during monsoon, and four wells largely abstract groundwater perennially. Bank filtrate proportion in a well is not dependent on its distance from the lake. Also, more than one groundwater stream appears to be contributing to the well field. Such anomalous hydrology is likely due to hydrogeological heterogeneity in the landslide debris or drainage from fractures and faults in the underlying geology. The study shows that an LBF well in a landslide deposit can sustainably deliver water of drinking quality at a short distance of ~5 m and travel time of ~2 to 3 days from the lake.
Similar content being viewed by others
Introduction
Bank filtration, a natural filtration process, leads to attenuation of pathogens, organics, turbidity, as well as many inorganic contaminants in the surface water as it passes through the bank aquifer (Ray et al. 2003). Bank filtration is applicable to both river banks and lake banks. However, there are a few differences between lake bank filtration (LBF) and river bank filtration (RBF). Due to low water movement in lakes, colmation layer at lake banks grows steadily. It is not disrupted by the seasonal changes in water flows, as in the case of river banks. Studies on the littoral zones of the Lake Tegel, Berlin (Germany) have shown that the colmation layer, extending to a thickness of ~10 cm, is biologically active (Hoffmann and Gunkel 2011). Complete clogging of the layer does not take place due to aquatic organisms feeding on it.
Compared to RBF, very few water supply schemes based on LBF exist in the world in Germany, Finland, The Netherlands, Lithuania, Brazil, and India. In Berlin, Germany, LBF wells at Lake Tegel along with other aquifer filtration schemes furnish 70 % of the city’s water supply (Fritz et al. 2002). At the LBF schemes at Lake Tegel and Lake Wannsee in Berlin, bank filtrate takes a time of ~4 months to travel to shallow wells located ~100 m from the lake bank as estimated using stable isotopes (δ2H and δ18O), chloride, and boron as tracers (Massmann et al. 2008). In Dresden, Germany, an LBF scheme at Radeburg reservoir is used since 1986 for the city’s water supply which abstracts 90 % bank filtrate and 10 % groundwater (Chorus et al. 2001). Chorus et al. (2001) also showed that the bank filtration scheme filters 60–99 % of the microcystins concentration in the reservoir water. An LBF scheme installed at the bank of Lake Lagoa do Peri in Santa Catarina Island in Brazil is effective in removing 100 % of the phytoplankton and the cyanobacteria from the lake water (Romero et al. 2014). Miettinen et al. (1994) studied the LBF scheme on the bank of the Lake Kallavesi in Hietasalo Island, Finland. They observed removal of total organic carbon (TOC) from 12.1 mg/L in the lake water to 9.7 and 4.4 mg/L in wells located within 100 m and between 100 and 300 m, respectively, from the lake bank. Additionally, Finland also has LBF schemes at lakes Vihnusjärvi and Vesijärvi having TOC removal of >55 and >29 %, respectively (Kivimäki et al. 1998). In the Netherlands, water from the river Maas is pumped to a deep gravel extraction reservoir De Lange Vlieter, from where the water is abstracted through LBF wells (Juhász-Holterman et al. 1998). This process has the capacity to reduce pathogens by at least 3.5 logs in the water. In Lithuania, bank infiltrate from the Kaunas reservoir in the Kaunas City recharges the LBF well field (G. Jonkutė, personal communications, 2010). These schemes, however, are located in alluvial aquifers consisting of sands and gravels of varying geological ages.
An LBF scheme developed in 1990 on the bank of the lake Naini in Nainital town in the state of Uttarakhand, India exists in a landslide debris deposit rather than an alluvial aquifer. Landslide deposit as an aquifer has fundamental differences from alluvial aquifers. Alluvial aquifers have vertical stratification and horizontal long-range homogeneity. Landslide deposits, on the other hand, lack horizontal or vertical homogeneity. There is no geomechanical stratification, and the deposit consists of randomly distributed impermeable zones and permeable channels, whose location and connectivity may change with time (Matti 2008). Permeable zones are preferentially found closer to the sliding surface of the landslide. In addition, particles in the landslide deposits have higher heterogeneity in shapes and sizes than in alluvial aquifers. As a result, movement of water through the deposit is highly unpredictable.
An investigation by Dash et al. (2008) showed that the Nainital LBF scheme rendered water free from coliform. Total Coliform counts up to 5 × 105 MPN/100 mL in the lake water were completely eliminated during bank filtration. Nachiappan et al. (2002) analyzed stable isotopes of water in the various water sources in the region in 2000 and assessed average proportion of lake water in the LBF wells. Subsequently, additional tubewells have been commissioned, and the number of wells has increased from 5 to 7 and then to 12. However, one of the old wells was dismantled, and two wells have not been in regular operation due to functional difficulties. Thus, the scheme presently consists of nine functional tubewells.
With the extension of the LBF scheme, a necessity of investigating the entire well field was felt. A study was undertaken in 2012–2013 to assess the performance of LBF scheme in terms of the (1) spatio-temporal variations in the quality of the lake water and the well water and (2) the proportions of bank filtrate in the well waters. The evaluation is based on the analysis of the data on water quality and stable isotope (δ2H and δ18O) values of the waters.
Site description
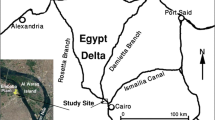
Lake Naini (29°23′N, 79°28′E) is located 1936 m above sea level in a geologically unstable region of the Kumaun Himalayas, India. It is surrounded by steep mountain ranges as shown in Fig. 1a. The Naini Fault that runs midway across the lake separates the two ranges. On the southeast end of the lake, the terrain slopes downhill and sluices are installed to control the water level in the lake. Mean retention time of water in the lake is ~1.16 years (AHEC 2002). Rainfall in the region mostly occurs during the monsoon season lasting from June to September. In the present study, however, the monsoon period has been defined from June to November to include the effects of post-rainfall sub-surface flows generated by the rains.
The geology of this tectonically active and landslide prone region has been extensively studied by many researchers (Valdiya 1988). The region has a hard rock geology consisting of folded bedrocks. The mountains consist of slates, marls, carbonates, limestones, dolomites, sandstone, and conglomerates (Valdiya 1980). The northwest bank of the lake (Fig. 1a) comprises of substantial debris deposit from several landslides that occurred between 1867 and 1924, before which this area was a part of the lake. An exploratory drilling done near the LBF wells revealed that the deposit consists of irregular collapsed boulders and rock fragments, shale/slate, and sand approximately to a depth of about 36 m followed by a clay layer (Ashraf 1978). Rest of the lake periphery largely consists of bedrocks. There are no alluvial deposits in the region. Groundwater moves largely along numerous faults, fractures and pore spaces in the rocks, often emerging as perennial or seasonal springs. The LBF scheme at Nainital is a unique example of bank filtration in non-alluvial aquifers with fault- and fracture-ridden bedrock geology beneath. Dash et al. (2008) have described the bank filtration site and the local geology in detail.
Nainital was one of the first towns in India to have a piped water supply system developed in 1898 with water drawn from local springs. In 1955, the use of lake water was started to augment the existing water supply. Over the years, anthropogenic activities in the lake catchment resulted in severe eutrophication of the lake (Pant et al. 1981). Therefore, a treatment plant was established in 1985 for purification of the lake water for water supply. Subsequent to installation of the LBF scheme, direct pumping of the lake water and its purification were discontinued.
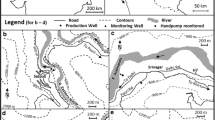
With the addition of new wells and decommissioning of the old wells since then, the current LBF well field consists of nine wells, shown in Fig. 1b. Of the nine wells, W1, W2, W3, W4, and W7 are within 15 m of the lake bank, and W5, W6, W8, and W9 are between 40 and 95 m from the lake bank. The wells are located close to the Naini fault. The ground level of the well field is gently sloping with an altitude difference of 1.5 m between the wells W4 and W9. Wells W4 and W5 are the oldest existing wells drilled in years 1999 and 2000, respectively. Wells W6 and W7 were drilled in 2006, and the remaining wells W8, W9, W1, W2, and W3 were drilled during 2008–2009. The wells have a diameter of 30 cm with depths of 26.7 m for W4 and between 33 and 38 m for the other wells. Filter screens are located between 10 and 26 m below ground level in W4 and between 13 and 37 m below ground levels in other wells. Wells abstract water at a rate ranging from 23 to 40 L/s. The abstracted water is stored and chlorinated before supply.
Grain size distribution of the aquifer material obtained during drilling of the well W6 yielded hydraulic conductivities at different depths ranging from 200 to 400 m/day (Dash et al. 2008). Based on hydraulic conductivities, travel time of the bank filtrate to the wells W4 and W5 was estimated to be 1–2 days and 11–19 days, respectively. Similar values of hydraulic conductivities of ~275 m/day were obtained for the wells W1 and W3 and ~430 m/day for the well W5 using pumping tests with Boulton’s analysis (Sandeep 2011).
Among the other water sources, many of the springs in the region have dried over the years. Pardadhara (PD) is one of the perennial springs running for more than 100 years and is located close to the LBF wells. In recent years, it has a very low discharge particularly during the non-monsoon period. Water from this spring is also used for municipal supply when needed and if the discharge is sufficient. Sukhatal, a rainy season lake, is located uphill along the Naini fault, which soon dries after the monsoon season is over. A tubewell has been drilled near Sukhatal to abstract groundwater for local supply, henceforth called Sukhatal Tubewell (ST).
Lake Naini and its catchment have been the focus of environmental studies and conservation efforts. There have been studies on the lake water and sediment geochemistry by Das et al. (1995), Chakrapani et al. (2002), and Purushothaman et al. (2012), and its water balance by AHEC (2002). Since 2007, there have been improvements in sewage and solid waste management in the lake catchment and hypolimnetic aeration has been implemented in the lake. The aeration system includes 30 aeration disk modules installed at the lake bottom (Kumar 2008). The modules release compressed air mixed with small amounts of ozone at a pressure of ~310 kPa. Dissolved oxygen (DO) levels of >3–4 mg/L are maintained up to the lake bottom by controlling the aeration rate. Gupta and Gupta (2012a, b) have studied the water quality and ecosystem changes in the lake during introduction of the aeration system.
Materials and methods
Sample collection
Sampling campaigns to collect both lake and well water samples were organized almost monthly from April, 2012 to November, 2013. Lake water samples from four locations, L1–L4, as shown in Fig. 1a were collected from ~0.5 m below the lake surface. Water samples were also collected from the nine tubewells W1–W9, as shown in Fig. 1b, while the pumps were in operation. PD and ST water samples were collected from Jan 2013 to Nov 2013 and from Feb 2013 to Nov 2013, respectively. A portable multi-parameter probe (HQ40d, Hach, Loveland, USA) was used for on-site measurements of electrical conductivity (EC), pH, temperature and dissolved oxygen (DO). Samples for off-site analysis at IIT Roorkee for the other parameters were collected, preserved, and transported as per the Standard Methods (APHA et al. 2005). Polypropylene bottles of 15 mL capacity were used to collect and transport the analysis of stable isotopes. It was ensured that no air was entrapped in the head space of the bottles.
Sample analysis
Water quality analysis at IIT Roorkee was carried out as per the methods prescribed in Standard Methods (APHA et al. 2005). The water samples were first filtered through 0.22 µm size filter (Millipore, GVWP) for the estimation of dissolved ions and organics. Cations and anions were analyzed using an ion chromatograph (Metrohm, AG-861) while titrimetric method was used for the determination of alkalinity. TOC-VCSN Total Organic Carbon Analyzer (Shimadzu) and DR5000 spectrophotometer (HACH) were used for the determination of DOC (dissolved organic carbon) and UV-A (UV absorbance at 254 nm), respectively. Total and fecal coliform counts were analyzed using the multiple-tube fermentation technique.
Stable isotope analysis was carried out using GV Isoprime Dual Inlet Isotope Ratio Mass Spectrometer at National Institute of Hydrology, Roorkee (India). Samples (400 µL) were equilibrated for 7 h with CO2 for δ18O analysis and with H2 reference gas and Pt catalyst for δ2H analysis. The precisions were ±0.1 ‰ and ±1 ‰ for δ18O and δ2H, respectively. The delta (δ) values reported are with respect to Vienna Standard Mean Ocean Water (VSMOW).
Results and discussion
Water withdrawn from a well at a lake bank is either filtered lake water or groundwater or mixture of lake water and groundwater. The first task was to ensure the connectivity of the wells with the lake through sub-surface flow and to know the proportions of bank filtrate in the water samples of different wells in different seasons. An assessment of the proportions of bank filtrate in the well waters has been done by analyzing (1) EC, (2) stable isotopes, and (3) ionic compositions of the lake water, well waters, and the ground water sources. The attenuation of the contaminants has been assessed in terms of changes in total and fecal coliform counts, DOC, turbidity, and UV absorbance of the water samples.
EC and stable isotopes data
An insignificant variation in the water quality and stable isotope characteristics of the lake water samples collected from four locations (L1–L4 as marked in Fig. 1a), was observed. The lake water is vertically mixed due to hypolimnetic aeration. Keeping these observations in view, subsequent sampling of the lake water was done at location L1 only. The δ18O values for the lake water were higher than the water samples from PD and ST by ~1 ‰ (Table 1). The EC values of the lake water were lower than those of the other water samples.
Drying of springs over years, and lack of hand pumps or wells in the area limited the possible groundwater sampling locations. The ground water generally does not exhibit much temporal variation whereas surface and sub-surface water quality reveals the seasonal changes. Therefore, groundwater has been fixed on the basis of the seasonal variability in EC, isotopic signatures and ionic concentrations.
The δ18O of water from all the wells is in between the δ18O values of the lake water and the PD and ST samples (Table 1). These values suggest the mixing of the bank filtrate with the PD and/or ST water in the RBF wells. The average EC values of the waters from the nine wells ranging from 622 to 847 μS/cm (Table 1) are higher than that of the lake water. However, the EC of the water from the wells W5, W6, W8, and W9 is consistently 100–150 µS/cm higher than the PD and ST samples. Therefore, on the basis of the EC values, mixing of lake water with PD or ST water in W5, W6, W8, and W9 is ruled out. Among these, the EC of water from the W9 remained nearly unchanged from monsoon to non-monsoon periods, thus displaying characteristics of groundwater.
The plot of δ18O vs. δ2H values further showed the seasonal and spatial differences in the various well waters (Fig. 2). The isotopic values of the waters were close to the local meteoric water line, determined based on the rainfall isotopic data from Kumar et al. (2010), and the global meteoric water line (Rozanski et al. 1993). During non-monsoon months, the lake and well waters showed a characteristic enrichment of 18O without a significant change in 2H concentration. Evaporation enrichment of surface water leads to variation of δ18O and δ2H values along local evaporation lines with slopes typically between 4.0 and 6.0 (Gibson et al. 2008). Isotopic enrichment of 18O without change in δ2H can occur due to oxygen exchange with carbonate or silicate minerals, particularly in deep groundwater and at higher temperatures (Gat 2010). The observed variation in δ18O vs. δ2H values for non-monsoon samples (along lines with slopes <4.0) indicates a combined effect of evaporative enrichment of lake water and deeper sub-surface 18O isotopic enrichment in groundwater, likely from carbonate minerals present in the Nainital geology.
The monsoon and non-monsoon values of W9 water are different from the corresponding values of lake water. The isotopic pattern of the waters from W5 and W6 is similar to W9. However, W1, W3, W7, W8 and ST waters have analogous isotopic signatures but are lighter than the lake water in terms of both δ18O and δ2H. Their isotopic values are scattered equally on the two sides of the meteoric line. On the other hand, water samples of W6, W5, and W9 were isotopically lighter than the lake mostly in terms of δ18O only and their data points are mostly left of the meteoric line. This dissimilarity in patterns suggests that more than one stream of groundwater contribute to the well field and their contributions are different in each well. These streams might have a common origin—most likely recharge from the precipitation—but have undergone different hydrogeological trajectories before reaching the bank aquifer. The δ18O and δ2H values of W2 and W4 are similar to the lake water in both monsoon and non-monsoon periods, suggesting these two wells to be predominantly drawing the bank filtrate.
Inorganic ions in the waters
EC of water samples is presented in Table 1. Concentrations of major ions Ca2+, Mg2+, SO4 2−, and HCO3 − contributing to EC are shown in Fig. 3. The amounts of ions Na+, K+, Cl−, and NO3 − are low, adding little to EC. The K+ concentrations ranged from 3 to 9 mg/L in the water from the lake and wells. Concentrations of Na+ were in the range 7–14 and 9–29 mg/L in the lake and well waters, respectively. The Cl− concentration of the lake and well waters, however, fluctuated in the range 6–9 and 7–21 mg/L, respectively. The NO3 − concentration in the lake and the wells ranged from 2 to 16 mg/L and <1 to 35 mg/L, respectively. In the PD and ST samples, NO3 − concentrations ranged from 9 to 14 mg/L.
Perusal of data in Fig. 3 indicates that ionic concentrations of the well waters are more than in the lake water. The average ionic concentrations of the water from the wells W5, W6, W8, and W9 are also more than in the waters from PD and ST. This observation could be due to the mineralization of water during sub-surface flow of the water or mixing of groundwater other than the water from PD and ST. Moreover, based on ionic concentrations as seen in Fig. 3, the well waters could be categorized into three groups. Waters from Group I wells W2 and W4 have Ca2+ and SO4 2− concentrations almost similar, and Mg2+ and alkalinity (HCO3 −) slightly higher, than the lake water values. Waters from Group II wells W1, W3, and W7 have Ca2+, Mg2+, and alkalinity more than the lake water during the summer and monsoon months but comparable to that of lake water during other times. Water from Group III wells W5, W6, W8, and W9 has much higher ionic concentrations than the lake water, as well as PD and ST—similar to the trends in the EC values (Table 1).
The average Langelier Saturation Index (LSI) of lake water was estimated to be 0.65 with range −0.2 to 1.6, and that of the wells was 0.35 with range −0.1 to 0.8. The average LSI values of PD and ST were 0.14, and 0.49, respectively. These values indicate that the lake, the wells, as well as the ground waters are largely saturated or supersaturated, and there is no significant CaCO3 mineralization in the well field aquifer. These data also supported that the groundwater reaching wells W5, W6, W8 and W9 has a component different from the ground waters at PD and ST, because PD and ST waters mixing with the lake water alone could not yield the increased ionic concentrations in the waters from these wells.
It was observed that, in general, Ca2+ and Mg2+ concentrations in the lake and the bank filtrate decreased during summer and monsoon but remained nearly constant in the groundwater. Such temporal variation was not noticed in the concentrations of the other ions. Indian drinking water standard IS:10500 (2012) has specified desirable limits of 75 mg/L for Ca2+ and 30 mg/L for Mg2+. In addition, the standard has also specified the maximum permissible limits of 200 mg/L and 100 mg/L for Ca2+ and Mg2+, respectively, in the absence of an alternate source. The concentrations of Ca2+ and Mg2+ ions often exceeded the prescribed desirable values. However, the concentrations were less than the permissible limits.
The observed ionic composition of the waters is consistent with the findings of Nachiappan and Kumar (1999) and Chakrapani et al. (2002), who ascribe the presence of limestone, dolomite, and other carbonates as source for Ca2+, Mg2+, and HCO3 − ions; silicate minerals as source for Na+ and K+ ions; and oxidation of sulfides in pyritiferous shale and dissolution of gypsum deposits as source for SO4 2− ions. Lack of any spatial pattern in the NO3 − concentrations suggested that these were caused by localized contamination from anthropogenic activities. The molar ratio of Ca2+ to Mg2+ in the water samples from all the sources was found to be around 0.75. This ratio is indicative of leaching from predominantly Mg2+-containing minerals such as dolomite. During May 2013, most water quality parameters showed an abrupt change in values. The Ca2+/Mg2+ molar ratio in all water sources suddenly increased to about 1.3. The parameters reached their steady-state values within next 2 months. It is not possible to ascribe the cause of this abrupt change based on the present data.
Proportion of bank filtrate in the wells
Perusal of data presented in Table 1, Figs. 2 and 3, and subsequent discussion indicate the following:
-
1.
The EC of the lake water is less than that of the well waters.
-
2.
The EC of the well waters varies from well to well. Also, the EC of the waters from the wells during monsoon has been found to be more than that in the non-monsoon.
-
3.
Waters from W5, W6, and W8 exhibit seasonal variation and are chemically similar.
-
4.
The EC of the water from the well W9 does not vary from monsoon to non-monsoon. Isotopic signature of W9 water is consistently different than the lake water.
-
5.
Waters from W2 and W4 are isotopically and chemically similar to the lake water.
-
6.
The δ18O values of waters from W1, W3, W5, W6, and W7 in monsoon is less than in non-monsoon.
Based on these observations, isotopic characteristics of W9, PD, and ST water samples have been considered as representative of the ground water streams contributing to the well field. Although, possibility of other groundwater streams contributing to the wells cannot be ruled out. Based on these assumptions, a comparison of the monsoon to non-monsoon variation in average isotopic and EC values of the three groups of wells (categorized on the basis of the ionic concentrations) with respect to lake and groundwater samples is presented in Fig. 4.
The first group of wells, W2 and W4 with water isotopically and chemically similar to the lake almost throughout the year continuously draw bank filtrate. The second group of wells, W1, W3, and W7 draw large proportions of bank filtrate during the non-monsoon period and largely groundwater during the monsoon period. These results are unusual particularly because the wells W1, W2, and W3 are located at similar distances from the lake bank, have similar depths and filter screen locations (variation ~1 m), and yet abstract such different proportions of bank filtrate. The third group of wells W5, W6, and W8 (along with W9) with isotopic and chemical characteristics largely similar to the groundwater abstracts predominantly groundwater throughout the year. These results suggest the presence of a highly localized and channeled groundwater flow close to these wells as postulated in Fig. 5.
Based on the average δ18O values, annual average bank filtrate proportions were estimated for the wells. The wells W2 and W4 abstracted ~80 % lake water, W1 and W7 abstracted 40–75 % bank filtrate annually. Interestingly, while variation of bank filtrate proportions in W3 was similar to W1 and W7, annual average proportion of bank filtrate in this well was ~30 % only. The wells W5, W6, and W8 abstracted <35 % bank filtrate. These proportions are shown with pie diagrams in Fig. 5.
Nachiappan et al. (2002), however, estimated higher proportion of bank filtrate (80 %) in the wells during monsoon and a lower percentage (20 %) during non-monsoon. This difference in results could be pointing to actual changes in the local hydrology in the lake bank aquifers over the years, as reflected in the differences in the isotopic values of the waters in the two studies. Their groundwater sample was average from the springs in the whole region. Most of these springs have dried by now, and the present analysis includes only three representative groundwater streams contributing to the well field.
Wells W2 and W4 should be pumped more to obtain water with lower Ca2+ and Mg2+ concentrations. Wells W5, W6, W8, and W9 should be pumped only in cases of higher demand of water.
Attenuation of coliforms, turbidity, and dissolved organics
Since the introduction of hypolimnetic aeration, coliforms, turbidity, and organics have significantly decreased in the lake water. The coliform counts in the lake have decreased by two orders of magnitude to low levels of ~2000 MPN/100 mL. A decrease in the coliform counts of the lake is also likely to be due to disinfection by ozone used in the aerators. During the study period, DO of the lake surface water ranged from 4.5 mg/L in July to 9.0 mg/L in January. The turbidity of the lake ranged between 1.7 and 5.5 NTU. The mean UV-A of the lake was 0.035 cm−1 with a mean DOC of 3.1 mg/L—much lower than the DOC of most lakes in the world (Sobek et al. 2007).
Total coliform counts of <2 MPN/100 mL were observed in most of the well waters except for contamination of ~10–30 MPN/100 mL in a few wells in the summer months (Fig. 6). The PD samples invariably responded positively to the total coliform test possibly due to local contamination from anthropogenic activities. The wells did not have any significant fecal contamination, except once or twice in W7, W8, and W9. Such occasional contamination is addressed by chlorination of the abstracted water before supply.
Turbidity and organics were consistently lower in the wells than the lake throughout the year. As shown in Fig. 7a, turbidity in well waters never exceeded 5 NTU and mostly remained around 0.2 NTU. The average DOC and UV-A values of the well waters were ~1 mg/L and less than 0.03 cm−1, respectively (Fig. 7b, c). The organics present in the lake water are reduced by 19–76 % during filtration through the aquifer material. This percentage is comparable to organics removal in other LBF systems in the world (Miettinen et al. 1994; Kivimäki et al. 1998). The SUVA values of the well waters ranged from 0.1 to 2.3 L mg−1 m−1, while that of the lake water ranging from 0.7 to 2.3 L mg−1 m−1, suggesting a slight decrease in aromatic compounds in the wells compared to the lake (Weishaar et al. 2003).
The study demonstrates that it is possible to obtain high-quality bank filtrate at very short distances and short travel times from a lake bank throughout the year.
Sustainability of pumping at the Nainital LBF site
The water balance study was undertaken by the AHEC (2002) in 2001. It was reported that of the lake outflow, 26 % was pumped by the LBF wells (at that time), 56 % was flow through sluices, 8 % was lost through evaporation, and 10 % was through downhill springs. Pumping by LBF wells can be increased to a maximum of 82 % of the lake outflow, when the outflow through sluices would decrease substantially. Beyond this limit, water abstraction would reduce the water quantity in the lake and the groundwater level in the region. Therefore, based on volumetric balance calculations, with a lake volume of 5.91 × 106 m3 and a mean retention time of 1.16 years, pumping through LBF wells can remain sustainable up to an abstraction rate of ~13.9 × 103 m3/day. Current water abstraction by the wells varies between 12 × 103 and 16 × 103 m3/day (depending on seasons) of which ~40 to 50 % is bank filtrate on an average. Therefore, current operation of the LBF wells is sustainable, but in future, the quantity of bank filtrate abstraction can only be increased by up to a factor of 2.
Conclusions
Present study shows that the proportions of bank filtrate in the LBF wells at Naini lake bank do not strictly decrease with the distance from the lake. In addition, there are different seasonal variations in different wells. Two wells located within 15 m of the lake bank abstract ~80 % bank filtrate almost throughout the year. However, three other wells located between 10 and 15 m of the lake bank and close to the first two wells abstract 50–80 % bank filtrate during the non-monsoon period but mostly groundwater during monsoon. Four wells located between 40 and 100 m of the lake bank abstract <35 % bank filtrate almost throughout the year. Average proportions of the bank filtrate in the wells are shown in Fig. 8. Possible reasons for such unusual hydrology are the heterogeneity in the landslide debris aquifer and the presence of Naini fault and other fractures very close to the well field. This study has shown that combined use of stable isotope and water quality data can help in understanding of complex hydrological systems.
This study also shows that a landslide deposit is effective at removing coliforms, turbidity, and organics from surface water. The LBF scheme in Nainital abstracts mostly drinking quality water at short infiltration distances of <5 m and travel times of ~2 to 3 days. A few events of coliform contamination in some of the wells are observed for brief periods, possibly caused by local contamination from infiltrating surface water, which are addressed by chlorination. The LBF pumping in Nainital is sustainable with respect to water balance of the lake.
The waters in the region have Ca2+, Mg2+, HCO3 −, and SO4 2− as the predominant ions. Ca2+ and Mg2+ concentrations are slightly higher than the desirable limits but lower than the maximum permissible limits for drinking water. Wells W2 and W4 drawing the maximum bank filtrate should be pumped more to obtain water with lower Ca2+ and Mg2+ than the groundwater. Abstraction from other wells can be regulated according to the seasonal changes to get a maximum amount of bank filtrate with a lower amount of Ca2+ and Mg2+.
References
AHEC (2002) Detailed project report on conservation and management plan for Nainital Lake. AHEC/2002, Alternate Hydro Energy Centre, Indian Institute of Technology Roorkee, Uttaranchal (now Uttarakhand), India
APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, DC
Ashraf Z (1978) A geological report on the drilling explorations carried out for the study of Nainital hill slope stability, Nainital district, U.P. Report of Geological Survey of India, Northern Region, Lucknow, India
Chakrapani GJ (2002) Water and sediment geochemistry of major Kumaun Himalayan lakes, India. Environ Geol 43(1):99–107
Chorus I, Schlag G, Heinze R, Pütz K, Kruspe U (2001) Elimination of microcystins through bank filtration at the Radeburg reservoir. In: Chorus I (ed) Cyanotoxins, occurrence, causes, and consequences. Springer, Berlin, pp 226–228
Das BK, Singh M, Van Grieken R (1995) The elemental chemistry of sediments in the Nainital lake, Kumaun Himalaya, India. Sci Total Environ 168(1):85–90
Dash RR, Mehrotra I, Kumar P, Grischek T (2008) Lake bank filtration at Nainital, India: water quality investigations. Hydrogeol J 16(6):1089–1099
Fritz B, Sievers J, Eichhorn S, Pekdeger A (2002) Geochemical and hydraulic investigations of river sediments in a bank filtration system. In: Dillon P (ed) Management of aquifer recharge for sustainability. Swets and Zeitlinger, Lisse, pp 95–100
Gat JR (2010) Isotope hydrology: a study of the water cycle. Imperial College Press, London, p 138
Gibson JJ, Birks SJ, Edwards TWD (2008) Global prediction of δA and δ2H−δ18O evaporation slopes for lakes and soil water accounting for seasonality. Global Biogeochem Cy 22: GB2031
GoogleMaps (2014) http://maps.google.com/. Accessed 1 Oct 2014
Gupta R, Gupta PK (2012a) a) The impact of artificial circulation on phytoplankton community on a freshwater eutrophic lake. J Ecophysiol Occup Health 12:1–20
Gupta R, Gupta PK (2012b) b) The effect of artificial de-stratification on the water quality of a freshwater high altitude lake. J Ecophysiol Occup Health 12:27–41
Hoffmann A, Gunkel G (2011) Bank filtration in the sandy littoral zone of Lake Tegel (Berlin): structure and dynamics of the biological active filter zone and clogging processes. Limnologica 41(1):10–19
IS:10500 (2012) Indian Standard: Drinking Water Specifications. Publication of Bureau of Indian Standards, New Delhi, India
Juhász-Holterman MHA, Peters JH, Geerts JJGM (1998) Artificial recharge of a lake excavated for gravel extraction. In: Peters JH et al (eds) Artificial recharge of groundwater. Balkema, Rotterdam, pp 237–242
Kivimäki AL, Lahti K, Hatva T, Tuominen SM, Miettinen IT (1998) Removal of organic matter during bank filtration. In: Peters JH et al (eds) Artificial recharge of groundwater. Balkema, Rotterdam, pp 107–112
Kumar A (2008) Hypolimnetic withdrawal for lake conservation. In: Sengupta M, Dalwani R (eds) Proceedings of Taal 2007: The 12th World Lake Conference, pp 812–818
Kumar SU, Kumar B, Rai SP, Sharma S (2010) Stable isotope ratios in precipitation and their relationship with meteorological conditions in the Kumaon Himalaya, India. J Hydrol 391(1–2):1–8
Massmann G, Sültenfuß J, Dünnbier U, Knappe A, Taute T, Pekdeger A (2008) Investigation of groundwater residence times during bank filtration in Berlin: a multi-tracer approach. Hydrol Process 22(6):788–801
Matti B (2008) Geological heterogeneities in landslides: characterization and flow modelling. D.Sc. Thesis, École Polytechnique Fédérale De Lausanne, Switzerland
Miettinen IT, Martikainen PJ, Vartiainen T (1994) Humus transformation at the bank filtration water plant. Water Sci Technol 30(10):179–187
Nachiappan RP, Kumar B (1999) Proceedings IUGG 99 Symposium HS4. Birmingham 258:215–219
Nachiappan RP, Kumar B, Manickavasagam R (2002) Estimation of sub-surface components in the water balance of Lake Nainital (Kumaun Himalaya, India) using environmental isotopes. Hydrol Sci J 47:41–54
Pant MC, Gupta PK, Pande J, Sharma PC, Sharma AP (1981) Aspects of water pollution in Lake Nainital, U.P., India. Environ Conserv 8:113–117
Purushothaman P, Mishra S, Das A, Chakrapani GJ (2012) Sediment and hydro biogeochemistry of Lake Nainital, Kumaun Himalaya, India. Environ Earth Sci 65(3):775–788
Ray C, Melin G, Linsky RB (2003) Riverbank filtration: improving source-water quality. Kluwer Academic Publishers, Dordrecht
Romero LG, Mondardo RI, Sens ML, Grischek T (2014) Removal of cyanobacteria and cyanotoxins during lake bank filtration at Lagoa do Peri, Brazil. Clean Techn Environ Policy 16:1133–1143
Rozanski K, Araguás-araguás L, Gonfiantini R (1993) Isotopic patterns in modern global precipitation. In: Swart PK, Lohmann KC, McKenzie K, Savin S (eds) Climate change in continental isotopic records. American Geophysical Union, Washington DC, pp 1–36
Sandeep C (2011) Lake bank filtration induced transport of microorganisms. M.Tech. Dissertation, Department of Civil Engineering, Indian Institute of Technology Roorkee, India
Sobek S, Tranvik LJ, Prairie YT, Kortelainen P, Cole JJ (2007) Patterns and distribution of dissolved organic carbon: an analysis of 7500 widely distributed lakes. Limnol Oceanogr 52(3):1208–1219
Valdiya KS (1980) Geology of Kumaun Lesser Himalaya. Gyānodaya Prakashan, Nainital
Valdiya KS (1988) Geology and natural environment of Nainital hills, Kumaun Himalaya. Gyānodaya Prakāshan, Nainital
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37(20):4702–4708
Acknowledgments
This study was conducted as a part of the project ‘Saph Pani–Enhancement of natural water systems and treatment methods for safe and sustainable water supply in India’, funded by the European Commission (Grant Agreement No. 282911). The authors are grateful to Uttarakhand Jal Sansthan for their co-operation in this study. Laxmi Das, Anand Bharti, Sandeep C., and Pooran Patwal at IIT Roorkee and F. Mütze and S. Zschau at HTW Dresden are thanked for help in the investigations. The mobility of F. Mütze and Sören Zschau was funded by the project (no. 27110509) ‘NachhaltigeSiedlungswasserwirtschaft (Sustainable Urban Water Management)’ funded by the German Academic Exchange Service’s (DAAD) programme “A New Passage to India” (2009–2011). Soma Kumari is thanked for discussions on LBF that helped immensely in writing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, A., Singh, H., Ahmed, F. et al. Lake bank filtration in landslide debris: irregular hydrology with effective filtration. Sustain. Water Resour. Manag. 1, 15–26 (2015). https://doi.org/10.1007/s40899-015-0001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-015-0001-z