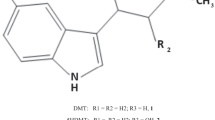
Abstract
Apomorphine is now recognized as the oldest antiparkinsonian drug on the market. Though still underused, it is increasingly prescribed in Europe for patients with advanced Parkinson’s disease (PD) with motor fluctuations. However, its history is far from being limited to movement disorders. This paper traces the history of apomorphine, from its earliest empirical use, to its synthesis, pharmacological development, and numerous indications in human and veterinary medicine, in light of its most recent uses and newest challenges. From shamanic rituals in ancient Egypt and Mesoamerica, to the treatment of erectile dysfunction, from being discarded as a pharmacological tool to becoming an essential antiparkinsonian drug, the path of apomorphine in the therapeutic armamentarium has been tortuous and punctuated by setbacks and groundbreaking discoveries. Throughout history, three main clinical indications stood out: emetic (gastric emptying, respiratory disorders, aversive conditioning), sedative (mental disorders, clinical anesthesia, alcoholism), and antiparkinsonian (fluctuations). New indications may arise in the future, both in PD (palliative care, nonmotor symptoms, withdrawal of oral dopaminergic medication), and outside PD, with promising work in neuroprotection or addiction.
Similar content being viewed by others
Apomorphine has a long and tortuous path in the therapeutic armamentarium, with numerous indications in human and veterinary medicine. |
The controversy that apomorphine aroused among clinicians in the past (and in some ways, continues among neurologists) can be explained by the lack of controlled studies and its affiliation to morphine. |
There are three main indications for apomorphine: emetic, sedative, and antiparkinsonian. |
This old drug needs to be reconsidered by clinicians and will benefit from current galenic and technical advances, both in Parkinson’s disease and in other indications. |
1 Introduction
Apomorphine is recognized as the oldest antiparkinsonian drug and is increasingly prescribed across Europe [1,2,3]. It is currently used by subcutaneous injection, as needed (pen) or continuously (continuous subcutaneous apomorphine infusion or CSAI), to treat motor fluctuations in patients with advanced Parkinson’s disease (PD) [4]. If apomorphine was first synthesized in the middle of the 19th century, its history goes back much further. This old drug has followed a tortuous path in and out of the armamentarium, shaped by a blend of mystical beliefs and stereotypes, and punctuated by setbacks and groundbreaking discoveries (Table 1). In this article, we trace the rich and eventful history of apomorphine, bringing to light some of the forgotten names associated with it. There is a copious literature on apomorphine, but much of it consists of uncontrolled studies and case reports. We therefore adopted a descriptive and categorizing approach, reporting the essential archival literature on apomorphine published between 1845 and January 2018, and discussing it in light of contemporary issues. We undertook a nonsystematic database (MEDLINE, NCBI, PubMed, Google Scholar, JSTOR, BnF Gallica and the Internet Archive) search for French and English articles with the terms ‘apomorphine’, ‘apomorphia,’ and ‘sulfomorphide’ (Fig. 1). This analysis was supplemented with pragmatic searches using references and authors’ names found in these articles.
Three main indications stood out from all the rest: emetic, antiparkinsonian, and sedative. It was quite astonishing to see the amount of controversy that apomorphine aroused among clinicians in the past and, in some ways, continues among neurologists.
2 Waterlilies and Aporphine Alkaloids: An Insight into Empirical Pharmacology
Though apomorphine is known as a synthetic product, anthropologists, ethnobotanists and pharmacologists have tracked down an early use of it in ancient civilizations, with striking cross-cultural similarities in Nymphaea cults between Mesoamerica and Egypt, where mind-altering plants were part of the religious and healing systems [5, 6].
The blue waterlily, Nymphaea caerulea Savigny, grows in the still waters of northern and central Africa [5]. An Osirian emblem [5, 6] by virtue of its natural cycle, and a symbol of the continual renewal of life, N. caerulea was extensively used as a motif in funerary art (pharaohs’ tomb frescoes, jewelry, funerary ceramics) and as an ornament for the dead (wreaths of dry flowers were found in the mummified remains of Ramses II and Tutankhamun) [5, 7, 8]. Spread across Egyptian sites of religious activity between the 5th and 22nd dynasties [5, 9], the portrayal of buds or partially opened flowers is commonly associated with representations of mandrakes and opium poppies, suggesting mystical properties [5, 10], though there is no direct evidence that N. caerulea was actually made into a narcotic preparation [5, 7]. Its use in religious rituals is, however, reported in the Papyrus of AniFootnote 1 [5, 11], and depicted in the rite of purifying the nostrils, a ritual of great importance in Egypt [5]. Reserved for the highest castes, N. caerulea was also reportedly used as an aphrodisiac [11, 12].
Nymphaea ampla (Salisbury) de Candolle, recognizable for its white flowers, thrives in Mesoamerican freshwater lakes [5]. Its use in religious and healing activities was first hinted at by Marlene Dobkin de Rios (cultural anthropologist), José-Luis Díaz (medical researcher), and William A. Emboden (botanist), collecting evidence from chemistry, botany, anthropology, art, literature, and history of religion [5]. The white waterlily figures prominently in pre-Columbian Mesoamerican iconography [10]. Abundant clues to its entheogenic properties are found in poems, ceramics, carved stone reliefs and frescoes, where it is constantly associated with representations of mythical beings, mushrooms,Footnote 2 human anatomy and death symbols [10, 13, 14, 56]. During the Classic Mayan period, N. ampla was associated with fertility and presumably used by a priestly caste to induce shamanic ecstasies and hallucinations [5, 7, 9, 14]. The Dresden Codex features the Waterlily Jaguar,Footnote 3 god of the Mayan netherworld, frequently associated with libations, drinking vessels and hallucinogenic enemas [5, 7, 13]. More recently, a recreational use of fresh, raw rhizomes of N ampla was reported in the seventies in some areas of Chiapas, Mexico [7], and aporphine alkaloids (apomorphine-like alkaloids, nupharine, nupharadine [5, 7, 9]) were subsequently isolated from its rhizomes and roots [5].
The abundant archeological and ethnographic evidence of the use of these Nymphaea for ritualistic purposes in both civilizations [5, 9] warrant further analytical investigation to assert the presence of apomorphine among the isolated alkaloids.
3 The 19th and 20th Centuries: From Apomorphine Synthesis to Understanding its Pharmacology
3.1 Synthesis
The credit for discovering apomorphine goes to the Finnish chemist Adolf Edvard Arppe, who synthesized it in 1845, in the form of its sulfate, by heating morphine with a slight excess of sulphuric acid [15, 16]. Soluble in caustic alkalinities, turning from white to green on keeping [15,16,17], the resultant product was classified as an amid by Auguste Laurent and Charles Frédéric Gerhardt and named sulfomorphide [16]. Of note, this different name may explain why Arppe’s work tends to be overshadowed by that of Augustus Matthiessen and Charles Romley Alder Wright, who are usually regarded as the first to have synthesized apomorphine. In 1850, Thomas Anderson also obtained apomorphine by heating codeine with sulfuric acid [18,19,20], but none of this work seemed to stir much interest at the time. It was not until 1869, and Matthiessen and Wright’s chemical investigation of the opium alkaloids, that apomorphine became known to the medical community [21, 22]. Heating morphine with concentrated hydrochloric acid, the two chemists synthesized apomorphine hydrochlorate, the salt currently used in therapeutics [21], and named it apomorphia, to emphasize both its origin and difference from morphineFootnote 4 [21, 22]. It, too, turned green when exposed to air [21, 22]. Carrying out their research, they quickly found other ways of synthesis, repeating Arppe’s experiment or heating codeine with hydrochloric acid or zinc chloride [17, 23].
3.2 Milestones Along the Road to Understanding the Pharmacology of Apomorphine
3.2.1 Chemical Formula and Structure
In 1869, apomorphine formula (C17H17NO2) was found to be the morphine formula (C17H19NO3) minus a water molecule (H2O) [21], but its exact structure was only elucidated in 1902 [24]. In 1965, Anton Marie Ernst was the first to draw attention to the structure–effect relationship between apomorphine and dopamine receptors, stressing the importance of the chemical structure (OH– group or OCH3– group at the para-position) in relation to the pharmacological effect [25,26,27]. The key part of the molecule in terms of its interaction with dopamine receptors was later found to be the dihydroxytetrahydroaminonapthalene moiety [28] or catechol moiety [29]. X-ray methods determined the absolute configuration of the active molecule to be 6aR [30].
3.2.2 Physiological Effects of Apomorphine
Though apomorphine was ignored for several decades following its discovery, it started to attract considerable interest after 1869, and soon became the subject of extensive clinical research, as evidenced by the abundant literature of the 19th century, particularly in the United Kingdom, France, and Germany [19, 20, 22]. The few pharmacological similarities between morphine and apomorphine were immediately noted [21, 31]: “in spite of its name, [apomorphine] is no more like morphine than sawdust is like sugar” [32]. Within a month of its synthesis, apomorphia was addressed to Dr Samuel Jones Gee for investigation [21, 22]. A wide range of physiological effects would be revealed, both in animals and humans, with interspecies and intraspecies variations in susceptibility and dose-related differential effects [20, 22, 33, 34]. The most salient characteristic was its nonirritant, certain, sustained but transitory emetic properties, when administered either orally or subcutaneously in dogs and humans [22]. “Lassitude, weakness, frequent headaches, constant nausea, and occasional sudden attacks of vomiting” were even noticed during the drug’s preparation, owing to absorption through the skin [22, 31]. Stereotyped motor behaviors (dogs), excitement, pupil dilation, and epileptiform convulsions (cats) and sedation (humans) were also reported [22]. The earliest pharmacological studies, attributed to Vincent Siebert and Erich Harnack [33, 34], confirmed Gee’s findings and highlighted cardiovascular, hemodynamic, and temperature changes [33, 34], as well as stereotyped behavior in a variety of susceptible species (Table 2 [22, 33, 34]).
3.2.3 Locus of Action
Early experiments led Gee to believe that apomorphine-induced symptoms were “referable to the nervous system” [22]. Subsequent experiments exploring movement, emesis, motivated behavior, respiratory control, and sensation strengthened the hypothesis of a powerful action on the central nervous system (CNS), in multiple brain centers, and not on the nervus vagus [9, 19, 20, 27, 33, 46,47,48]. Harnack emphasized two brain centers in particular: vomiting (‘Brechcentrum’) and respiratory (‘Respirationscentrum’) [33]. In dogs, Lazarus Thumas neutralized apomorphine-induced emesis by destroying a portion of the medulla oblongata [49], whereby the respiratory center was separated from the vomiting one [19, 50]. The powerful emetic action of apomorphine was attributed to a selective effect on the chemoreceptor trigger zone [32]. Experiments with decerebrate dogs showed that vomiting was induced when apomorphine was administered either parenterally or locally to the medullary chemoreceptor trigger zone, whereas depression of muscular rigidity only appeared when apomorphine was administered parenterally [50, 51]. Caesar Hans Amsler was the first to strongly associate the action of apomorphine with the striatum, abolishing the apomorphine-induced pecking syndrome in pigeons with the surgical removal of the corpora striata [39]. These findings were later strengthened by the induction of compulsive gnawing behavior in rats after the implementation of crystalline apomorphine in the dorsal part of the caudate nucleus and/or globus pallidus [26]. Pretreatments depleting central catecholamine stores or inhibiting monoamine oxidase failed to modify the gnawing-provoking action of apomorphine [27]. Ernst therefore concluded that apomorphine has a direct dopamine-like effect on receptors and may act as a substitute in the case of dopamine deficiency in the extrapyramidal structures (e.g., PD [27]), hence providing a post hoc explanation for the discovery by Robert S. Schwab and colleagues [52, 53]. These experiments spurred the resurgence of basic and clinical research on apomorphine [29] and have since become a classic in the literature [53]. Apomorphine-induced stereotyped behavior in rodents was then attributed to the direct stimulation of postsynaptic dopamine receptors [54]. Typical stereotypies were provoked by intrastriatal microinjection of apomorphine in nialamide-pretreated rats, but blocked by a prior injection of chlorpromazine (dopaminergic antagonist), thus supporting the idea that apomorphine acts on dopaminergic receptors located in the striatum [55]. The paradoxical suppression of chorea and dyskinetic movements in humans led to the hypothesis that apomorphine acts primarily on presynaptic receptors [56]. Finally, the discovery that apomorphine does not solely act on the dopaminergic pathway was prompted by the observation that its effect on tremor can also be mediated by serotonergic neurons [57]. It is now known that apomorphine also acts on serotonergic and adrenergic receptors [58, 59].
3.3 Apomorphine as an Investigative Tool
The history of apomorphine is intrinsically linked to the development of pharmacology, neurobiology, and neurochemistry. By the 1960 s, apomorphine had become one of the most intensively studied drugs in neuropsychopharmacology and behavioral therapy, largely based on Ernst’s work [25,26,27, 53, 60]. It has been frequently used as a pharmacological probe for investigating central dopaminergic neurotransmission and screening new drugs (Fig. 2).
Being a nonspecific dopaminergic agonist [58, 59], apomorphine can be used as a pharmacological tool and is suitable for stimulating dopamine receptors and exploring dopaminergic pathways [61]. Apomorphine-induced stereotyped behaviors in experimental animals have been used as an index of central dopaminergic activity and has contributed to the understanding of dopaminergic systems [62]. Apomorphine-induced yawning in rats and humans [29] is a biological marker for central dopamine system alterations (enhanced responsiveness is found in patients with migraine or heroin addiction) and a measurement of central dopamine function [28, 63, 64]. Although further studies are needed to assess the usefulness of apomorphine as a biological marker for substance-dependence disorders (changes in dopaminergic sensitivity), the first reports seem rather promising [65, 66]. Apomorphine has also been used as an endocrine challenge test to assess central dopaminergic responsiveness in Wilson’s disease [67] and to investigate hypothalamic-adenohypophyseal function [68]. Because it can increase human growth hormone (GH) secretion and decrease prolactin secretion [29, 68], apomorphine was used to assess the adequacy of GH secretion in serum [68, 69] and investigate the effects of psychotropic drugs [29]. Apomorphine-induced changes in the GH response have been reported in schizophrenia [29, 70], anorexia nervosa, PD [29, 71], depression [29], Huntington’s chorea, tardive dyskinesia, and obsessive-compulsive disorder [29, 72].
Apomorphine-induced behaviors have been used through animal models to test compounds for therapeutic properties and to define comparable potencies. Apomorphine-induced emesis has been used to assess antiemetic agents [73] and antagonism of apomorphine-induced stereotypy has been widely used in the study of neuroleptics and the screening of drugs for psychotropic activity, including haloperidol and risperidone [42, 69, 74–76].
4 From the 19th to the 21st Century: Treating Animals and Humans
In almost 150 years, an extensive body of literature has been published on apomorphine, attesting to its use in a broad range of conditions (Fig. 3). However, most of it consists of case reports and uncontrolled studies.
4.1 Veterinary Medicine
Following Gee’s experiments, apomorphine was used as an emetic in cases of poisoning and esophageal foreign bodies in dogs, pigs, and cats to rapidly induce forced emesis (even if cats do not respond consistently to it [76]). Today, apomorphine remains the emetic of choice in dogs, administered either parenterally or topically in the conjunctival sac of the eye [77]. Other veterinary uses are now confined to history books: expectorant in dogs, pica (licking sickness) in cattle [76], or acute strychnine poisoning in dogs [78, 79]. Conflicting reports are found for the latter, some highlighting positive results through emesis and spasm relaxation [78, 79] while others describe inefficacy [78, 80].
4.2 Clinical Uses Across Time
As soon as apomorphine had been deemed suitable for subcutaneous injections [17, 22, 81], it started to be used in human medicine, albeit initially as quite an expensive treatment [19]. Interestingly, apomorphine has always had its supporters and detractors, regarded by some as “remarkable” [79] but by others as “curious, dangerous, obsolete and antiquated” [82, 83]. Thus, although it was studied and prescribed for a wide range of medical conditions, it remained “insufficiently recognized” [84] and considered a “sort of taboo” subject [32] by “doctors who w[ould] cheerfully inject cobra venom or malaria into their patients [but] refuse[d] to inject apomorphine”, as stated provocatively by Dent [32]. Taken together, poor study design, uncontrolled studies, small sample sizes, calls for caution following alarmist case reports (exceptional cardiovascular collapse in children and adults), untoward accidents (confusion between morphine and apomorphine), poor conservation, and differences in doses (resulting in the consecutive stimulation of pre- and/or post-synaptic dopaminergic receptors), forms (amorphous or crystallized), routes of administration, and even purity between suppliers in the early 20th century, not to mention the heterogeneity of patient populations in psychiatric investigations, may explain the discrepant and unreliable results found in the literature, and thence the failure to translate these findings into standard clinical care, as well as the general distrust surrounding apomorphine [19, 20, 29, 47, 48, 62, 82, 85,86,87,88].
4.2.1 Emetic Potency
One of the best documented effects of apomorphine is emesis. It is striking to note that what is now viewed as a troublesome but treatable side effect in the treatment of PD [4] remained the main indication of apomorphine for a whole century, overshadowing all other uses.
4.2.1.1 Gastric Emptying
Apomorphine is the most effective known centrally acting emetic drug [60], and was the drug of choice for any condition requiring prompt emptying or cleaning of the stomach and ejection of esophageal foreign bodies [19, 89, 90] until the 1970's [91]. The possibility of subcutaneous administration was greatly appreciated, particularly when oral administration was impracticable (refractory child or adult, coma or delirium) [90]. Poisoning was usually treated with ipecac syrup, but as apomorphine could produce rapid, nonirritant, efficient and controlled emesis, its use, either orally, hypodermically, or rectally, became extremely widespread [22, 31, 48, 86, 89, 90]. Many case reports advocated the use of apomorphine to treat various types of poisoning (opium, acid, mushroom, metal, or arsenic [86, 92, 93]), particularly for accidental poisoning in children [86, 92] or suicide attempts [94] and acute alcohol poisoning, being mentioned as early as 1869 in Dr Philip John Hensley’s experiments [22, 32]. Some clinicians were, however, reluctant to use it, owing to the difficulty of monitoring toxicity signs [95], as the vomiting was usually followed by sleep [32]. Anecdotal reports from the late 1800's show that apomorphine was also used to clean an ‘upset stomach’ [86] or to induce vomiting in cases of sunstroke [35], meningitis [96], and ardent fever [97]. From the mid-1950 s to the end of the 1970 s, apomorphine was given intravenously as a pre-anesthetic emetic in labor to prevent inhaled vomitus (a common cause of death from general anesthesia in obstetrics) [98,99,100], inducing complete emptiness of the stomach prior to analgesia. Though considered to be a good alternative to stomach tubes [98,99,100] and not deleterious to either the mother or the newborn [98,99,100], this approach was rejected by the majority of anesthesiologists, based on the observation that apomorphine did not always induce vomiting at the prescribed doses [101].
4.2.1.2 Respiratory Disorders
Used mainly as an oral, nasal, cutaneous, or subcutaneous expectorant [19, 31, 32, 81, 86], administered either alone or in combination with morphine, apomorphine was prescribed in the 19th century to reduce coughing in breathing difficulties or thick, tenacious mucus [81], as in winter cough (chronic bronchitis, bronchial catarrh), bronchorrhea [81, 86, 90], catarrhal laryngitis [90], pneumonia [31], dyspnea, hemoptysis [19, 86], and croup [86, 90]. It also relieved angina pectoris and asthma thanks to its relaxing effect, in combination with scopolamine [79, 90, 102]. The effect of apomorphine on dopaminergic control of the cough reflex was demonstrated in cats more than a century later [103].
4.2.1.3 Aversive Conditioning
Apomorphine was extensively used in the first half of the 20th century to induce aversive conditioning, through its emetic and sedative properties [32, 104]. The apomorphine aversion conditioning method consisted of provoking nausea and vomiting timed to the administration of the addictive substance, thereby inducing a conditioned repulsion. Its combination with emetine or caffeine was sometimes advocated [105]. As part of the conditioned reflex aversion treatment of alcoholism [32, 83, 105,106,107,108], apomorphine became an agent of choice in the mid-20th century across Europe and the US [32, 108], many decades after being a key component of the Keeley Cure, which was famous in the late 1800 s for inebriety [106]. John Yerbury Dent was a fervent advocate of apomorphine for treating alcoholism, on account of both its aversive capacity and its ability to prevent craving through an action in the brain [32, 60, 83, 105]: “this is one of the very few treatments in which the patient can expect a miracle” [32]. Walter Voegtlin, by contrast, was convinced that exact timing was essential for true Pavlovian conditioning, and discarded apomorphine because of its short duration and sedative effect, which he viewed as hindrances to true conditioning [105, 108]. Treatment duration varied between days and weeks, depending on addiction severity [60]. Conflicting results are found, for while a randomized, double-blind, clinical trial on the effects of oral apomorphine on alcohol post-intoxication symptoms [109] failed to yield any positive results, other studies reported high levels of success [84], amply relayed by William S. Burroughs (who extensively described his own experience and recovery in his books: Health Bulletin: APO-33, a metabolic regulator, 1965; The Naked Lunch, 1967), greater attendance in sober states for continuing treatment, and a “non-significant tendency to manage better both motorically and mentally” [110]. Alcohol addiction was not the only indication for apomorphine, as it was used in cases of morphine, heroin, barbital, methadone [9, 12, 40, 105, 109, 111], or tobacco [105, 111] addiction and also, shockingly, in homosexuality, to induce nausea concomitant to the presentation of male nude pictures, alongside electric shocks and psychotherapeutic techniques [112, 113].
4.2.2 Sedative and Hypnotic Effects
The sedative and hypnotic properties of apomorphine were first described by Gee and outlined by Charles J. Douglas [22, 104, 114]. Appearing at subemetic doses with a prompt, dependable and safe effect, they led to clinical applications for a variety of conditions characterized by excitement, anxiety and/or agitation [22, 82, 83, 114]. Following the observation that it was “of special value in all forms of mania” [114], apomorphine was deemed to be of “noteworthy value in the care of disturbed psychiatric patients” [84]. As early as 1912, Dr Francis Hare praised the use of hypodermic injections of apomorphine in a book where he listed three main uses: “(1) in maniacal or hysterical drunkenness; (2) during the paroxysm of dipsomania, in order to still the insistent craving for alcohol; and (3) in essential insomnia of a special variety” [115]. The emetic and sedative effects of apomorphine allowed inebriated patients to sleep calmly and awaken without hallucinations or delirium, and even in some cases without any desire for alcohol [32, 79, 82, 114, 115]. Over time, indications extended to other psychiatric conditions [32, 60, 79, 82, 83, 105, 107, 114,115,116,117], with abundant descriptions of cases that were satisfactorily treated with apomorphine (either alone or associated with scopolamine [107, 118]): mania [22], alcoholic mania [32, 79, 82, 114, 115], hysteria and hysteroid attacks [32, 47, 86, 119,120,121,122,123], alcoholic insomnia [107, 114, 115], schizophrenic excitement (sedative action, reduction of hallucinations and delusions [29, 32, 84, 124, 125]), paranoia [84], panic states of acute or alcoholic anxiety [32, 60, 105, 107, 115], anxiety associated with grieving, suicidal thoughts, agoraphobia, or melancholia [32], agitation linked to depression [32], delirium tremens (although caution was advised, owing to the depressing action of apomorphine [32, 79, 82, 114]), senile dementia [84], post-traumatic excitement [84], postoperative and postpartum excitement [82, 84, 126,127,128], acute hyperthyroidism crisis [84], barbiturate reactions, confusion and restlessness following electroshock treatment or diverse organic states [84], and even pediatric enuresis of nervous origin [79]. As the above series shows, apomorphine was long associated with the treatment of alcohol-related disorders [32, 104, 107, 114]. It was also used as a premedication for emergency surgeries, or during recovery from anesthesia complicated by an agitation produced either by scopolamine, alcoholism, delirium tremens or morphine addiction [82, 107, 126,127,128]. The use of apomorphine-potentiated scopolamine analgesia in labor, tested on thousands of women [128], was found to be safe for both mother and baby, did not interfere with the progress of labor, and even reduced the incidence of postpartum respiratory complications [126, 128]. However, this technique was not recommended as a routine procedure [127].
4.2.3 Treating Sexual Disorders
Spontaneous apomorphine-induced penile erections were incidentally reported during the treatment of alcoholics [116, 129], and subsequently confirmed in rats, human control participants, and impotent patients [29, 116, 117, 129,130,131,132,133], highlighting the involvement of central dopamine receptors in normal erectile function and its impairment in a subgroup of patients [132]. A throwback to the early use of apomorphine in ancient civilizations, sublingual (SL) [134] apomorphine was commercialized at the beginning of the 2000's as the first centrally acting agent to be approved for the treatment of erectile dysfunction. However, despite their efficacy, safety, and benefits to patients [58], Ixense® (Takeda) and Uprima® (Abbott) were removed from the market in 2004 and 2006 in the face of competition from PDE5 inhibitors.
Since then, apomorphine has been suggested in the treatment of female sexual dysfunction [135,136,137,138].
4.2.4 Treating Movement Disorders
Despite being the first dopaminergic agonist to be used in PD, apomorphine suffered decades of neglect in the field of movement disorders.
4.2.4.1 Chorea, Spasms, Convulsions and Other Movement Disorders
As early as 1870, a beneficial effect on choreic movements was observed in a child exhibiting chorea associated with rheumatic fever [31]. Surprisingly though, Pierce disregarded apomorphine as a treatment for movement disorders, stating that it was “only as an emetic that [he] would draw attention to it” [31]. These effects were later reproduced [139, 140] and apomorphine was mentioned as a treatment for acute chorea in the 1915 edition of The Practitioner’s Encyclopaedia of Medical Treatment. Sydenham’s chorea could likewise be alleviated with apomorphine [84], as could congenital choreoathetosis, spontaneous orofacial dyskinesia [56], and Huntington’s disease (HD) [141,142,143]. In HD, apomorphine decreases the intensity of chorea, and improves motor impersistence and the ability to suppress associated movements [143]. Those effects are counteracted by haloperidol and sulpiride [142]. More recently, a double-blind, randomized, crossover trial (N = 5) showed that continuous infusion of apomorphine produces a sustained improvement in choreic symptoms, without affecting depressive symptoms [144]. These results need to be confirmed by more studies, but they already suggest that continuous apomorphine infusion should be considered in some patients with HD [144]. Apomorphine improves tics in Tourette’s syndrome [145, 146]. In tardive dyskinesia, results are variable, with either improvements (dyskinetic movements being alleviated in some patients, presumably through presynaptic effects [56, 124, 147]), or no effect at all [37].
Subcutaneous injections of apomorphine were used in the past to prevent or curtail epileptic seizures [47, 86, 90, 139, 140], alcohol-induced convulsions, eclampsia, and puerperal convulsions [90]. It also transiently blocked photosensitive epileptic discharge [148]. Following successful cases, apomorphine was considered to be effective in controlling muscle spasms, including nervous spasms (hiccoughs [90, 139] or spasmodic torticollis [29, 45], reducing the “marked muscular and psychic overactivity occasionally seen after […] scopolamine or atropine” [82]. It has been credited with cases of recovery after ingestion of presumably lethal amounts of strychnine [78] or even counteracting the tetanic state [22, 90].
Growing evidence suggests that apomorphine (either as boluses or CSAI) effectively improves periodic leg movements in restless leg syndrome (RLS) [149,150,151]. This indication needs to be further explored, particularly with CSAI. To be noted, RLS is also a frequent symptom in PD [150].
4.2.4.2 Parkinson’s Disease
Dr. Edmond Weill was the first to suggest that apomorphine might be useful in tremor and PD [139], bolstered by his success in treating chorea. A forgotten clinical note in The Canadian Medical Association Journal reports a use of apomorphine to alleviate spasticity in patients with paralysis agitans as early as 1935 [79], long before Schwab’s experiments [52]. Its author felt “justified in [his] choice of a sedative which can, with perfect safety, be administered for long periods of time” [79]. Dordoni’s work on decerebrate rigidity in dogs [51] paved the way for Schwab’s first use of apomorphine in PD in 1951 [52]. Despite a marked (albeit short-lived) improvement in symptoms and Ernst’s suggestion that apomorphine could successfully replace dopamine in PD [26], interest in the drug as an agent for managing movement disorders almost vanished when Georges Cotzias and collaborators launched the levodopa era [152]. Decades after, double-blind controlled studies initiated by Cotzias and colleagues demonstrated that apomorphine was a valuable drug in the antiparkinsonian armamentarium [60, 152, 153], especially when combined with metoclopramid [154, 155], haloperidol [155], or domperidone [156] to block the induced nausea. Stibe et al. updated the use of apomorphine in PD by demonstrating its beneficial effect on Parkinsonian on–off fluctuations, opening the way to further uses [157]. Apomorphine has since been used in the diagnosis (assessment of levodopa responsiveness) and treatment of PD in Europe, either as a pen injection or through continuous infusion [1,2,3,4, 12, 58, 59, 158]. Despite abundant evidence of its efficacy in treating advanced PD and its positive impact on patients’ quality of life [159, 160], apomorphine remains underused and unavailable to many patients worldwide [158]. Once again, work needs to be done to promote its use and improve its access. The TOLEDO study (ClinicalTrials.gov Identifier: NCT02006121), confirming the efficacy and safety of apomorphine, is a useful first step [161].
5 Apomorphine and Its Newest Challenges: Where Do We Stand Now?
More than a century has passed since apomorphine was first synthesized, during which time it has been abundantly studied. Although it has already been administered in many conditions and through all possible routes (hypodermic, oral, rectal, inhalation, IV, intranasal, sublingual [162, 163]), there is still room for improvements and new discoveries [162].
5.1 Challenges in the Treatment of Parkinson’s Disease
If apomorphine is currently only indicated for the motor fluctuations that usually occur after several years of levodopa treatment, growing evidence suggests other potential uses in PD, particularly for nonmotor PD subtypes [164], young patients (EARLYPUMP study, ClinicalTrials.gov Identifier: NCT02864004) and in cases where oral dopaminergic medications are withdrawn. However, apomorphine therapy is not devoid of adverse events (e.g., cutaneous lesions) that need to be addressed in the future.
5.1.1 Withdrawal of Oral Dopaminergic Medication
Missing antiparkinsonian medication has various consequences, and not all patients can tolerate being off medication due to severe off-state pain and dystonia [165, 166]. Withdrawal of oral dopaminergic medication can occur during surgery (general surgery or deep-brain stimulation [DBS]) but also in many cases where oral administration is hindered: loss of consciousness, intestinal occlusion, acute infection, or even during terminal care. Anecdotal evidence, associated with a few reports of local neurosurgical practice, suggests that apomorphine could be particularly useful in such situations.
5.1.1.1 Surgery
PD patients are particularly at risk of complications when undergoing surgery, both during and after the procedure—this risk being related to the disease itself and/or to the omission of medication [167,168,169,170]. Being unable to ingest oral medication because of hindered oral administration or poor gastrointestinal (GI) tract absorption (postoperative ileus, delayed gastric emptying) has major consequences [167, 169]. Dopaminergic deprivation can precipitate the deterioration and/or worsening of motor symptoms (rigidity, bradykinesia, swallowing difficulties, or inability to clear oral and pulmonary secretions) and lead to perioperative complications [165, 169, 170] including confusion, aspiration pneumonia, postoperative respiratory failure, bacterial infection (urinary tract infection), deep-vein thrombosis, pulmonary embolism, postoperative GI motility abnormalities, neuroleptic malignant-like syndrome (rarely), falls, and a prolonged postoperative hospital stay [167,168,169,170]. A few studies [167,168,169,170] suggest that parenteral apomorphine (subcutaneous injections [168] or continuously [167]) could be a good option for avoiding suboptimal treatment and alleviating nonmotor off-period symptoms—particularly digestive and urinary autonomic symptoms [168]. By preventing symptom resurgence and postoperative complications, subcutaneous apomorphine may facilitate nursing care and hasten surgical recovery [168].
Dopaminergic deprivation (generally starting 72 h prior to surgery) and awake testing are usual in DBS procedures, but put patients at risk of neurological and respiratory deterioration [171] or severe off-medication motor symptoms [166, 171]. Data from a single-center (N = 72 [171]) and anecdotal reports (N = 3 [170]; N = 1 [166]) strongly suggest that apomorphine, administered either via a pump or through repeated injections, is helpful during awake DBS surgery for relieving patients’ discomfort [166, 170, 171]. In some centers, apomorphine has even been added to preoperative guidelines [170, 171]. Domperidone can be introduced 2 days prior to surgery to avoid nausea [171]. Though no data is currently available, apomorphine may also be considered as an emergency treatment (for instance with pen injection at a predetermined dose) in case of acute stimulator failure in DBS patients, thanks to its rapidity of action [171].
5.1.1.2 Others
Though data are still scarce (N = 2), apomorphine injections may be particularly useful in the management of acutely ill PD patients (i.e., chest infection or stroke) who are unable to take their usual oral dopaminergic medication and/or tolerate a nasogastric tube [169]. In certain cases, apomorphine can be administered in the acute phase without prior administration of domperidone [169].
A single case report highlighted the pragmatic use of apomorphine in a terminally ill patient (73-year-old man with a 14-year history of PD) unable to take oral medication [172]. Signs of discomfort were alleviated with the injection of apomorphine 2 mg, alongside rectal administration of domperidone [172]. More studies are needed to confirm those results, but subcutaneous apomorphine deserves to be considered in PD terminal and/or palliative care.
Apomorphine has also been anecdotally reported to successfully treat neuroleptic malignant syndrome following abrupt reduction of chronic levodopa treatment in PD patients [165, 173].
5.1.2 Nonmotor Symptoms
Although subcutaneous apomorphine efficacy on parkinsonian nonmotor symptoms has been recently reviewed [174], there has been scant research on this particular topic, though it is of paramount interest. Preliminary data are encouraging and need to be confirmed in controlled and randomized studies.
Improvements induced by an apomorphine pump in the gastrointestinal domain of the Non-Motor Symptoms Scale have been recently demonstrated in 43 patients [159], supporting the results of previous studies on swallowing disorders and anorectal dysfunction in PD [175,176,177]. A beneficial effect on urinary symptoms (e.g., nocturnal urinary frequency, incontinence) has also been reported [159, 178, 179], though effect on detrusor hyperreflexia is inconsistent [179]. Sexual disorders are common in PD, but fail to receive the recognition they deserve, considering their impact on quality of life for both patients and their partners [179]. SL apomorphine has been used to treat erectile dysfunction in the general population but, surprisingly, no study has been led among PD patients; research is therefore needed as SL apomorphine is safe to administer and may improve sexual dysfunction [179].
Sleep disorder and nocturnal disabilities are frequent and varied in PD [180]. There is growing evidence that apomorphine, administered through a pump, brings some improvement in patients’ overall condition [159], with reduced nocturnal awakenings, off periods, pain, dystonia, nocturia, periodic limb movements or RLS, and insomnia [150, 159, 180]. To address the question of continuous overnight infusions, a randomized, cross-over study is currently underway in France to explore the effects of CSAI on sleep disorders in insomniac parkinsonian patients (APOMORPHEE study, ClinicalTrials.gov Identifier: NCT02940912).
5.1.3 Overcoming Adverse Events
Cutaneous adverse events linked to needle-based apomorphine therapy remain one of the biggest challenges in PD and may explain some of the reluctance surrounding its use. Avoiding toxicity and/or developing new delivery strategies are the two main research areas of CSAI [162]. Studies to understand the mechanisms linked to subcutaneous toxicity and to develop novel apomorphine formulae are underway [181].
Creating new and more practical pump devices is another area where further improvements could be made. Alternative delivery strategies that are currently being investigated include inhaled or SL apomorphine, oral delivery, and patch pump technology [162, 181].
If conflicting results are found on the neuropsychiatric effect of apomorphine, growing evidence tends to suggest that it is safe, and could even be beneficial for mood and apathy [59, 159], as well as induce a decrease in visual hallucinations [182,183,184] caused by visual problems [183, 185], possibly through an action on the serotonin 2A receptor [183]. Further work is needed to better understand this phenomenon.
5.2 Advances in Neurochemistry: New Indications for the Future?
New neuromolecular probes are emerging on the properties of apomorphine, suggesting that its neuroprotective effects could be harnessed as a potential modifier of amyloid deposition [162, 186].
Decades after its use in aversive conditioning for smoking cessation [105, 111], growing evidence suggests that apomorphine could be repurposed for tobacco use disorder, through its action on dopaminergic and serotoninergic receptors, both key to tobacco dependence mechanisms [88]. Administering low doses of SL apomorphine during the quitting process could stabilize the dopaminergic system, reverse hypersensitivity to nicotine, modulate serotoninergic pathways and counteract reinforcement cues [88]. Moreover, apomorphine is able to block ethanol and nicotine responses in the larval zebrafish locomotion model, suggesting that it could be used in tobacco dependence treatment, especially in combination therapy [187]. Further work is needed, but these studies highlight the undeniable value of this pharmacological agent.
6 Conclusion
Apomorphine is a remarkable example of an old drug being rediscovered. Its peculiar pharmacological profile (nonspecific dopaminergic and nondopaminergic agonist [58, 59]) has made it one of the most intensively studied drugs, and it can be safely assumed that apomorphine will continue to intrigue scientists and clinicians in years to come. Thanks to technical and galenic development, it is destined to become a key feature of future therapeutic strategies, and rightly so.
Change history
08 May 2018
In the original publication, the name of the author in T.
Notes
‘The Papyrus of Ani’ is referred to as The Book of the Dead.
A well known sacred route to ecstasy in Mayan civilization.
A jaguar wearing a waterlily headdress.
The prefix ‘Apo’ meaning ‘away from’.
References
Sharma JC, Macnamara L, Hasoon M, Vassallo M. Diagnostic and therapeutic value of apomorphine in Parkinsonian patients. Int J Clin Pract. 2004;58(11):1028–32.
Antonini A. Apomorphine and levodopa infusion therapies for advanced Parkinson’s disease. J Mov Disord. 2009;2(1):4–9. https://doi.org/10.14802/jmd.09002 Epub 2009 Apr 30.
Chaudhuri KRL, Rizos A, Sethi KD. Motor and nonmotor complications in Parkinson’s disease: an argument for continuous drug delivery? J Neural Transm (Vienna). 2013;120(9):1305–20. https://doi.org/10.1007/s00702-013-0981-5.
Trenkwalder C, Ray CK, García RPJ, LeWitt P, Katzenschlager R, Sixel-Döring F, et al. Expert consensus group report on the use of apomorphine in the treatment of parkinson’s disease—clinical practice recommendations. Parkinsonism Relat Disord. 2015;21(9):1023–30. https://doi.org/10.1016/j.parkreldis.2015.06.012.
Emboden WA. Transcultural use of narcotic water lilies in ancient Egyptian and Maya drug ritual. J Ethnopharmacol. 1981;3(1):39–83.
Emboden WA, de Rios DM. Mayan-Egyptian uses of water lilies (Nymphaceae) in shamanic ritual drug use. In: Meyer, George G, Blum, Kennet, Cull, John G, editors. Folk medicine and herbal healing. Geneseo: Springfield; 1981. p. 275–86.
Emboden WA. The sacred narcotic lily of the nile: nymphaea caerulea. Econ Bot. 1978;32:395. https://doi.org/10.1007/BF02907935.
Kandeler R, Ullrich WR. Symbolism of plants: examples from European-Mediterranean culture presented with biology and history of art. J Exp Bot. 2009;60(9):2461–4. https://doi.org/10.1093/jxb/erp166 (Epub 2009 Jun 3).
Taba P, Lees A, Stern G. Erich Harnack (1852–1915) and a short history of apomorphine. Eur Neurol. 2013;69(6):321–4. https://doi.org/10.1159/000346762 (Epub 2013 Mar 14).
Emboden WA. The mushroom and the water lily: literary and pictorial evidence for Nymphaea as a ritual psychotogen in Mesoamerica. J Ethnopharmacol. 1982;5(2):139–48.
Bertol E, Fineschi V, Karch SB, Mari F, Riezzo I. Nymphaea cults in ancient Egypt and the New World: a lesson in empirical pharmacology. J R Soc Med. 2004;97(2):84–5.
Stern G. Apolaustic apomorphine. Pract Neurol. 2013;13(5):335–7. https://doi.org/10.1136/practneurol-2012-000432 (Epub 2013 Mar 13).
McDonald A, Stross B. Water lily and cosmic serpent: equivalent conduits of the maya spirit realm. J Ethnobiol. 2012;32(1):74–107. https://doi.org/10.2993/0278-0771-32.1.74.
Carod-Artal FJ. Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurologia. 2015;30(1):42–9. https://doi.org/10.1016/j.nrl.2011.07.003.
Arppe AE. Ueber eine merkwürdige Veränderung des Morphins durch Schwefelsäure. Liebig’s Annalen der Chemie und Pharmacie 1845;LV:96.
Laurent A, Gerhardt C. On sulphomorphide and sulphonarcotide, derivatives from morphia and narcotina (1848) Philosophical Magazine Series 3, 33:223, 396-397, https://doi.org/10.1080/14786444808646128.
Matthiessen A. Researches into the constitution of the opium bases. Part II.—On the action of hydrochloric acid on codeia. Proc R Soc Lond (1868–1869);17:460-462.
Anderson T. On the constitution of codeine and its product of decomposition. Trans R Soc Edinb. 1853;20:57–86.
Bourgeois JBV. De l’Apomorphine, recherches cliniques sur un nouvel émétique. A. Delahaye (Paris), 1874, pp61 ark:/12148/bpt6k6138108d (in French).
David C. Contribution à l’étude physiologique du chlorhydrate d’apomorphine. L. Vincent (Lausanne), 1875, https://catalog.hathitrust.org/Record/100811374 (in French).
Matthiessen A, Wright CRA. Researches into the chemical constitution of the opium bases. Part I.—On the action of hydrochloric acid on Morphia. Proc R Soc Lond, 17 (1868–1869), pp. 455–460 http://www.jstor.org/stable/112441.
Gee S. On the action of a new organic base, apomorphia. Clin Soc Trans. 1869;2:166–9.
Matthiessen A, Burnside W. Researches into the chemical constitution of the opium bases. Part IV. On the action of chloride of zinc on codeia. Proc R Soc Lond. 1870;19:71–3.
Pschorr R, Jaeckel B, Fecht H. Über die Constitution des Apomorphins. Ber Dtsch Chem Ges. 1902;35:4377–92. https://doi.org/10.1002/cber.19020350496.
Ernst AM. Relation between the action of dopamine and apomorphine and their O-methylated derivatives upon the CNS. Psychopharmacologia. 1965;7(6):391–9.
Ernst AM, Smelik PG. Site of action of dopamine and apomorphine on compulsive gnawing behaviour in rats. Experientia. 1966;22(12):837–8.
Ernst AM. Mode of action of apomorphine and dexamphetamine on gnawing compulsion in rats. Psychopharmacologia. 1967;10(4):316–23.
Pinder RM, Buxton DA, Green DM. On the dopamine-like action of apomorphine. J Pharm Pharmacol. 1971;23(12):995–6.
Lal S. Apomorphine in the evaluation of dopaminergic function in man. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12(2–3):117–64.
Giesecke J. The absolute configuration of apomorphine. Acta Cryst. 1977;B33:302–3. https://doi.org/10.1107/S0567740877003458.
Pierce FM. Notes on apomorphia. Br Med J. 1870;1:204. https://doi.org/10.1136/bmj.1.478.204.
Dent JY. Apomorphine in the treatment of anxiety states, with special reference to alcoholism. B J Int. 1934;32:65–88. https://doi.org/10.1111/j.1360-0443.1934.tb05016.x.
Harnack E. Ueber die Wirkungen des Apomorphins am Säugethier und am Frosch. Arch Exp Pathol Pharmakol. 1874;2:254–306.
Siebert V: Untersuchungen über die physiologischen Wirkungen des Apomorphin. Inaugural-Dissertation zur Erlangung der Doctorgrades. Dorpat, Druck von Heinrich Laakmann, 1871.
Tomlinson Murphy. Report on the use of apomorphia in Sunstroke. Ind Med Gaz. 1879;14(11):307–10.
Colpaert FC, Van Bever WF, Leysen JE. Apomorphine: chemistry, pharmacology, biochemistry. Int Rev Neurobiol. 1976;19:225–68. https://doi.org/10.1016/S0074-7742(08)60705-9.
Levy MI, Davis BM, Mohs RC, Kendler KS, Mathé AA, Trigos G, Horvath TB, Davis KL. Apomorphine and schizophrenia. Treatment, CSF, and neuroendocrine responses. Arch Gen Psychiatry. 1984;41(5):520–4.
Montastruc P, Damase-Michel C, Montastruc JL. Apomorphine potentiates vagal bradycardia. Eur J Pharmacol. 1989;166(3):511–4.
Amsler C. Beiträge zur Pharmakologie des Gehirns. Naunyn-Schmiedebergs Arch Exp Pathol Pharmakol. 1923;97:1–14.
Kuschinsky K. An anthology from Naunyn-Schmiedeberg’s archives of pharmacology. E. Harnack (1874): Ueber die Wirkungen des Apomorphins am Säugethier und am Frosch. Archiv für experimentelle Pathologie und Pharmakologie 2: 254–306 (On the effects of apomorphine in mammals and frogs). In: Naunyn-Schmiedeberg’s Archives of Pharmacology; 373, 6; 387-389; Naunyn-Schmiedeberg’s Archives of Pharmacology by Springer-Verlag, Berlin/Heidelberg; 2006. https://doi.org/10.1007/s00210-006-0089-7.
Rampin O, Jérôme N, Suaudeau C. Proerectile effects of apomorphine in mice. Life Sci. 2003;72(21):2329–36.
Nymark M. Apomorphine provoked stereotypy in the dog. Psychopharmacologia (Berlin). 1972;26:361–8.
Goiny M, Uvnäs-Moberg K. Effects of dopamine receptor antagonists on gastrin and vomiting responses to apomorphine. Naunyn-Schmiedeberg’s Arch Pharmacol. 1987;336:16. https://doi.org/10.1007/BF00177745.
Montastruc JL, Llau ME, Senard JM, Tran MA, Rascol O, Montastruc P. A study of tolerance to apomorphine. Br J Pharmacol. 1996;117(5):781–6.
Pomerantz SM. Apomorphine facilities male sexual behavior of rhesus monkeys. Pharmacol Biochem Behav. 1990;35(3):659–64. https://doi.org/10.1016/0091-3057(90)90304-Z.
Chouppe. Quelques recherches sur le mode d’action des vomitifs les plus employés. Lyon Med. (1875) volume XVIII, p168 ark:/12148/bpt6k6483216v (in French).
Jones T. Hypodermic or Subcutaneous Medication. Br Med J. 1885;2(1291):581–7.
Pouchet G. Leçons de pharmacodynamie et de matière médicale Deuxième série, Hypnotiques (sulfonal, trional, hydrate d’amylène, paraldéhyde, uréthane). Modificateurs intellectuels (alcool, opium, chanvre indien). O. Doin (Paris), 1901, (in French).
Thumas L. Ueber das Brechcentrum und über die Wirkung einiger pharmakologischer Mittel auf dasselbe. Virchows Arch. 1891;123:44–9.
Borison HL, Wang SC. Physiology and pharmacology of vomiting. Pharmacol Rev. 1953;5(2):193–230.
Dordoni F. Sugli effete dell’associazione morfina-apomorfina nel cane. II. L’apomorfina e l’ipertono da decerebrazione. Boll Soc Ital Boil Sper. 1948;24:231–3.
Schwab RS, Amador LV, Lettvin JY. Apomorphine in Parkinson’s disease. Trans Am Neurol Assoc. 1951;56:251–3.
Cools AR. Commentary On: “Mode of action of apomorphine and Dexamphetamine on Gnawing compulsion in rats. Psychopharmacology. 2001;158(3):222–3 (Psychopharmacologia 1967;10:316-323).
Andén N-E, Rubenson A, Fuxe K, Hökfelt T. Evidence for dopamine receptor stimulation by apomorphine. J Pharm Pharmacol. 1967;19:627–9. https://doi.org/10.1111/j.2042-7158.1967.tb09604.x.
Ungerstedt U, Butcher LL, Butcher SG, Andén NE, Fuxe K. Direct chemical stimulation of dopaminergic mechanisms in the neostriatum of the rat. Brain Res. 1969;14(2):461–71.
Tolosa ES. Letter: paradoxical suppression of chorea by apomorphine. JAMA. 1974;229(12):1579–80.
Braham J, Sarova-Pinhas I, Goldhammer Y. Apomorphine in Parkinsonian tremor. Br Med J. 1970;3(5725):768.
Ribarič S. The pharmacological properties and therapeutic use of apomorphine. Molecules. 2012;17(5):5289–309. https://doi.org/10.3390/molecules17055289.
Auffret M, Drapier S, Vérin M. Pharmacological insights into the use of apomorphine in Parkinson’s disease: clinical relevance. Clin Drug Investig. 2018. https://doi.org/10.1007/s40261-018-0619-3.
Halvorsen KA, Martensen-Larsen O. Apomorphine revived: fortified, prolonged, and improved therapeutical effect. Int J Addict. 1978;13(3):475–84.
Costall B, Naylor RJ, Neumeyer JL. Differences in the nature of the stereotyped behaviour induced by aporphine derivatives in the rat and in their actions in extrapyramidal and mesolimbic brain areas. Eur J Pharmacol. 1975;31:1–16. https://doi.org/10.1016/0014-2999(75)90072-2.
Sourkes TL, Lal S. Apomorphine and its relation to dopamine in the nervous system. In: Agranoff BW, Aprison MH, editors. Advances in Neurochemistry. Heidelberg: Springer; 1975. p. 247–99. https://doi.org/10.1007/978-1-4757-4395-1.
Blin O, Azulay JP, Masson G, Aubrespy G, Serratrice G. Apomorphine-induced yawning in migraine patients: enhanced responsiveness. Clin Neuropharmacol. 1991;14(1):91–5.
Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez-Turet M. The apomorphine test: a biological marker for heroin dependence disorder? Addict Biol. 2002;7(4):421–6.
Casas M, Guardia J, Prat G, Trujols J. The apomorphine test in heroin addicts. Addiction. 1995;90(6):831–5.
Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez-Turet M. The apomorphine test: a biological marker for heroin dependence disorder? Addict Biol. 2002;7(4):421–6.
Frankel JP, Hughes A, Lees AJ, Stern GM, Walshe JM. Use of apomorphine to test for dopamine responsiveness in Wilson’s disease. Lancet. 1989;334(8666):801–2.
Lal S, De la Vega CE, Sourkes TL, Friesen HG. Effect of apomorphine on human-growth-hormone secretion. Lancet. 1972;2(7778):661.
Colpaert FC, Van Bever WF, Leysen JE. Apomorphine: chemistry, pharmacology, biochemistry. Int Rev Neurobiol. 1976;19:225–68. https://doi.org/10.1016/S0074-7742(08)60705-9.
Zemlan FP, Hirschowitz J, Garver DL. Relation of clinical symptoms to apomorphine-stimulated growth hormone release in mood-incongruent psychotic patients. Arch Gen Psychiatry. 1986;43(12):1162–7.
Friess E, Kuempfel T, Winkelmann J, Schmid D, Uhr M, Rupprecht R, Holsboer F, Trenkwalder C. Increased growth hormone response to apomorphine in Parkinson disease compared with multiple system atrophy. Arch Neurol. 2001;58(2):241–6.
Pitchot W, Hansenne M, Moreno AG, Ansseau M. Growth hormone response to apomorphine in obsessive-compulsive disorder. J Psychiatry Neurosci. 1996;21(5):343–5.
Proctor JD, Chremos AN, Evans EF, Wasserman AJ. An apomorphine-induced vomiting model for antiemetic studies in man. J Clin Pharmacol. 1978;18(2–3):95–9.
Depoortère R, Barret-Grévoz C, Bardin L, Newman-Tancredi A. Apomorphine-induced emesis in dogs: differential sensitivity to established and novel dopamine D2/5-HT(1A) antipsychotic compounds. Eur J Pharmacol. 2008;597(1–3):34–8.
Dias FR, de Matos LW, Sampaio Mde F, Carey RJ, Carrera MP. Opposite effects of low versus high dose haloperidol treatments on spontaneous and apomorphine induced motor behavior: evidence that at a very low dose haloperidol acts as an indirect dopamine agonist. Behav Brain Res. 2012;229(1):153–9.
Cerbelaud R. Manuel vétérinaire ou Formulaire des medications rationnelles et des remèdes secrets (conforme au Codex 1908). P. Cerbelaud (Paris), 1910, pp1290 (in French).
Cote DD, Collins DM, Burczynski FJ. Safety and efficacy of an ocular insert for apomorphine-induced emesis in dogs. Am J Vet Res. 2008;69(10):1360–5. https://doi.org/10.2460/ajvr.69.10.1360.
Haggard HW, Greenberg LA. Antidotes for strychnine poisoning. JAMA. 1932;98(14):1133–6. https://doi.org/10.1001/jama.1932.02730400011002.
Anderson DP. Apomorphia Hydrochloride. Can Med Assoc J. 1935;33(1):74–5.
Gold D, Gold H. Apomorphine as an antidote to strychnine poisoning. JAMA. 1933;100(20):1589–90. https://doi.org/10.1001/jama.1933.02740200023006.
Murrell W. On the action of apomorphine and apocodeine, with reference to their value as expectorants in the treatment of chronic bronchitis. BMJ. 1891;1:452–6 (No. 1574).
Rovenstine EA, Hershey SG. The utility of apomorphine in clinical anesthesia. Anesthesiology. 1945;6:574–9.
Parr D. Cardiovascular collapse during apomorphine treatment. Br Med J. 1956;2(4997):860–2.
Feldman F, Susselman S, Barrera SE. A note on apomorphine as a sedative. Am J Psychiatry. 1945;102:403–5. https://doi.org/10.1176/ajp.102.3.403.
Prevost JL. Note relative à un cas de collapsus inquiétant produit par l’apomorphine. Gazette hebdomadaire de médecine et de chirurgie. G. Masson (Paris), 1875, série 2, tome 12, p 20–22 (in French).
Bourneville DM, Bricon P. Manuel des injections sous-cutanées, 2ème édition revue et augmentée. A. Delahaye, E. Lecrosnier (Paris), 1885, (in French).
Levy MI, Davis BM, Mohs RC, Kendler KS, Mathé AA, Trigos G, Horvath TB, Davis KL. Apomorphine and schizophrenia. Treatment, CSF, and neuroendocrine responses. Arch Gen Psychiatry. 1984;41(5):520–4.
Morales-Rosado JA, Cousin MA, Ebbert JO, Klee EW. A critical review of repurposing apomorphine for smoking cessation. Assay Drug Dev Technol. 2015;13(10):612–22. https://doi.org/10.1089/adt.2015.680.
Verger T. De l’emploi de l’apomorphine pour l’extraction des corps étrangers de l’œsophage. Bulletin général de thérapeutique médicale et chirurgicale, 1878, no. 95, p 254–255. (in French).
Visanska SA. Apomorphine and its uses. Med Rec Weekly J Med Surg. 1898;54:15–16. https://archive.org/details/medicalrecordjou54newyuoft.
Rausten DS, Ochs MA. Apomorphine-naloxone controlled rapid emesis. J Am Coll Emer. 1973;2(1):44–5.
Maheu. Empoisonnement par les champignons. In: Bulletin général de thérapeutique médicale, chirurgicale, obstétricale et pharmaceutique (Paris, Doin) 1909, no. 157, p 540–544 (in French).
FitzPatrick V. A case of opium-poisoning: recovery. Br Med J. 1885;2:646 (No. 1292).
David. Prag. Med. Wochens, 1900, n°33 in Lyon medical 1902, no. 98, p 252. (in French).
MacLean WC Jr. A comparison of ipecac syrup and apomorphine in the immediate treatment of ingestion of poisons. J Pediatr. 1973;82(1):121–4.
Mason AL. Cerebro-spinal meningitis. Five cases. One, of the fulminant (foudroyant) type, treated by subcutaneous injection of apomorphia, morphine and atropine; recovery. One autopsy. Boston Med Surg J. 1184;110(6):121–6.
Miller WB. Apomorphine in ardent fever. BMJ. 1891;2:1309.
White RT. Apomorphine as an emetic prior to obstetric anesthesia; the prevention of inhaled vomitus. Obstet Gynecol. 1959;14(1):111–5.
Holdsworth JD, Furness RM, Roulston RG. A comparison of apomorphine and stomach tubes for emptying the stomach before general anaesthesia in obstetrics. Br J Anaesth. 1974;46(7):526–9.
Holdsworth JD. The place of apomorphine prior to obstetric analgesia. J Int Med Res. 1978;6(1):26–32.
Dinnick O. Discussion on Anæsthesia for Obstetrics an evaluation of general and regional methods [Abridged]. Proc R Soc Med. 1957;50(8):547–56.
Thomas J (1901) De l’asthme essentiel : son traitement. Imprimerie Le Bigot frères (Lille) (in French). http://gallica.bnf.fr/ark:/12148/bpt6k57118372.
Kamei J, Hukuhara T, Kasuya Y. Dopaminergic control of the cough reflex as demonstrated by the effects of apomorphine. Eur J Pharmacol. 1987;141(3):511–3.
Rosebrugh AM. A valuable discovery-a new hypnotic. Can Practitioner Rev. 1908;33:669–70.
Raymond MJ. The treatment of addiction by aversion conditioning with apomorphine. Behav ResTher. 1963;1(2–4):287–91.
Pershing HT. Mental therapeutics and the need of psychology in the medical curriculum. JAMA. 1902;39(10):551–3. https://doi.org/10.1001/jama.1902.52480360031001g.
Feldmann H. The ambulatory treatment of alcoholic addicts: a study of 250 cases. Br J Addict Alcohol Other Drugs. 1959;55(2):121–8.
Lemere F. Aversion treatment of alcoholism: some reminiscences. Br J Addict. 1987;82(3):257–8.
Wadstein J, Ohlin H, Stenberg P. Effects of apomorphine and apomorphine-L-dopa-carbidopa on alcohol post-intoxication symptoms. Drug Alcohol Depend. 1978;3(4):281–7.
Jensen SB, Christoffersen CB, Noerregaard A. Apomorphine in outpatient treatment of alcohol intoxication and abstinence: a double-blind study. Br J Addict. 1977;72:325–30.
Stern G. A case of excessive smoking. London: London Hospital Gazette; 1957. p. 144–5.
James B. Case of homosexuality treated by aversion therapy. Br Med J. 1962;1(5280):768–70.
McConaghy N. Subjective and penile plethysmograph responses following aversion-relief and apomorphine aversion therapy for homosexual impulses. Br J Psychiatry. 1969;115(523):723–30. https://doi.org/10.1192/bjp.115.523.723.
Douglas CJ. Alcoholism. N Y Med J. 1899;70:626–8.
Hare F. On alcoholism, its clinical aspects and treatment. London: JA Churchill; 1912.
Schlatter EKE, Lal S. Treatment of alcoholism with Dent’s oral apomorphine method. Q J Stud Alcohol. 1972;33(2):430–6.
Lal S, De la Vega CE. Apomorphine and psychopathology. JNNP. 1975;38:722–6.
Vessie PR. Scopolamin-apomorphia amnesia in psychiatry. Curr Res Anesth Analg. 1925;4(3):170–81.
Gowers WR. Epilepsy and other chronic convulsive diseases: their causes, symptoms and treatment. London: JA Churchill; 1881.
Bouzol M (1884) Relation d’une épidémie à phénomènes hystéro-choréiques observées à Albon (Ardèche) en 1882. Mémoires et comptes-rendus de la Société des Sciences Médicales de Lyon, tome XXIV, p 177–199 (in French).
Laurencin J. Effets thérapeutiques du chlorhydrate d’apomorphine en injections sous-cutanées dans l’hystéro-épilepsie. Lyon Méd. 1884;47:315–21 (in French).
Burgat C. Etude de deux cas d’hystéro-épilepsie. Lyon: Imprimerie Nouvelle; 1884 (in French).
Sainsbury H (1903) Apomorphine hydrochlorate: its use in mental affections. Merck’s Report.
Smith RC, Tamminga CA, Haraszti J, Pnadey GN, Davis JM. Effects of dopamine agonists in tardive dyskinesia. Am J Psychiatry. 1977;134(7):763–8. https://doi.org/10.1176/ajp.134.7.763.
Tamminga CA, Schaffer MH, Smith RC, Davis JM. Schizophrenic symptoms improve with apomorphine. Science. 1978;200(4341):567–8.
Hershenson BB, Brubaker ER. Scopolamine and apomorphine in labor. Am J Obstet Gynecol. 1947;53(6):980.
Sharpley HF Jr. Scopolamine in obstetrics; use in rapid births, premature births, and cesarean sections; control of restlessness with apomorphine in full term deliveries. Obstet Gynecol Surv. 1952;7(3):332–4.
White RR. The use of apomorphine with scopolamine in labor. Am J Obstet Gynecol. 1952;64(1):91–100.
Schlatter EK. Treatment of alcoholics with apomorphine using Dent’s method; a preliminary study. Montreal: Quebec Psychopharmacological Association; 1966.
Benassi-Benelli A, Ferrari F, Quarantotti BP. Penile erection induced by apomorphine and N-n-propyl-norapomorphine in rats. Arch Int Pharmacodyn Ther. 1979;242(2):241–7.
Lal S, Ackman D, Thavundayil JX, Kiely ME, Etienne P. Effect of apomorphine, a dopamine receptor agonist, on penile tumescence in normal subjects. Prog Neuropsychopharmacol Biol Psychiatry. 1984;8(4–6):695–9.
Lal S, Laryea E, Thavundayil JX, Nair NP, Negrete J, Ackman D, Blundell P, Gardiner RJ. Apomorphine-induced penile tumescence in impotence patients—preliminary findings. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:235–42.
Morales A. Apomorphine to Uprima: the development of a practical erectogenic drug: a personal perspective. Int J Impot Res. 2001;13(3):S29–34.
Heaton JP, Morales A, Adams MA, Johnston B, El-Rashidy R. Recovery of erectile function by the oral administration of apomorphine. Urology. 1995;45(2):200–6.
Bechara A, Bertolino MV, Casabé A, Fredotovich N. A double-blind randomized placebo control study comparing the objective and subjective changes in female sexual response using sublingual apomorphine. J Sex Med. 2004;1(2):209–14.
Caruso S, Agnello C, Intelisano G, Farina M, Di Mari L, Cianci A. Placebo-controlled study on efficacy and safety of daily apomorphine SL intake in premenopausal women affected by hypoactive sexual desire disorder and sexual arousal disorder. Urology. 2004;63(5):955–9.
Tarcan T, Siroky MB, Park K, Goldstein I, Azadzoi KM. Systemic administration of apomorphine improves the hemodynamic mechanism of clitoral and vaginal engorgement in the rabbit. Int J Impot Res. 2000;12(4):235–40.
Hamburger-Bar R, Rigter H. Apomorphine: facilitation of sexual behaviour in female rats. Eur J Pharmacol. 1975;32(02):357–60.
Weill E. De l’apomorphine dans certains troubles nerveux. Lyon Med 1884;47:411–8 (in French).
Vallender E. Berl Klin Woch. 1877;14:185–6. [in Vallender E. Apomorphine in Epilepsy. J Nerv Mental Disease. 1878;5(1):190].
Tolosa ES, Sparber SB. Apomorphine in Huntington’s chorea: clinical observations and theoretical considerations. Life Sci. 1974;15(7):1371–80.
Corsini GU, Onali PL, Masala C, et al. Apomorphine hydrochloride-induced improvement in huntington’s chorea: stimulation of dopamine receptor. Arch Neurol. 1978;35(1):27–30. https://doi.org/10.1001/archneur.1978.00500250031006.
Albanese A, Cassetta E, Carretta D, Bentivoglio AR, Tonali P. Acute challenge with apomorphine in Huntington’s disease: a double-blind study. Clin Neuropharmacol. 1995;18(5):427–34.
Vitale C, Marconi S, Di Maio L, De Michele G, Longo K, Bonavita V, Barone P. Short-term continuous infusion of apomorphine hydrochloride for treatment of Huntington’s chorea: a double blind, randomized cross-over trial. Mov Disord. 2007;22(16):2359–64.
Feinberg M, Carroll BJ. Effects of dopamine agonists and antagonists in Tourette’s disease. Arch Gen Psychiatry. 1979;36(9):979–85.
Lal S. Clinical studies with apomorphine. In: Corsini GU, Gessa GL, editors. Apomorphine and others dopaminomimetics. 2nd ed. New York: Raven Press; 1981. p. 1–11 (Clinical Pharmacology).
Tolosa ES. Modification of tardive dyskinesia and spasmodic torticollis by apomorphine. Possible role of dopamine autoreceptors. Arch Neurol. 1978;35(7):459–62. https://doi.org/10.1001/archneur.1978.00500310061013.
Quesney LF, Andermann F, Lal S, Peelevic S. Transient abolition of generalized photosensitive epileptic discharge in humans by apomorphine, a dopamine-receptor agonist. Neurology. 1980;30(11):1169–74.
Tribl GG, Sycha T, Kotzailias N, Zeitlhofer J, Auff E. Apomorphine in idiopathic restless legs syndrome: an exploratory study. J Neurol Neurosurg Psychiatry. 2005;76(2):181–5.
Reuter I, Ellis CM, Ray Chaudhuri K. Nocturnal subcutaneous apomorphine infusion in Parkinson’s disease and restless legs syndrome. Acta Neurol Scand. 1999;100(3):163–7.
Tings T, Stiens G, Paulus W, Trenkwalder C, Happe S. Treatment of restless legs syndrome with subcutaneous apomorphine in a patient with short bowel syndrome. J Neurol. 2005;252(3):361–3 (Epub 2005 Feb 23).
Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. N Engl J Med. 1967;276(7):374–9.
Düby SE, Cotzias GC, Papavasiliou PS, Lawrence WH. Injected apomorphine and orally administered levodopa in Parkinsonism. Arch Neurol. 1972;27(6):474–80.
Justin-Besançon J, Laville C. Action antiémétique du métoclopramide vis-à-vis de l’apomorphine et de l’hydergine. C R Soc Biol (Paris). 1964;158:723–7.
Corsini GU, Del Zompo M, Cianchetti C, et al. Therapeutical efficacy of a combination of apomorphine with sulpiride or metoclopramide in parkinsonism. Psychopharmacology. 1976;47:169. https://doi.org/10.1007/BF00735817.
Corsini GU, Del Zompo M, Gessa GL, Mangoni A. Therapeutic efficacy of apomorphine combined with an extracerebral inhibitor of dopamine receptors in Parkinson’s disease. Lancet. 1979;1(8123):954–6.
Stibe C, Lees A, Stern G. Subcutaneous infusion of apomorphine and lisuride in the treatment of parkinsonian on-off fluctutations. The Lancet. 1987;329:871 (No. 8537).
Henriksen T. Clinical insights into use of apomorphine in Parkinson’s disease: tools for clinicians. Neurodegener Dis Manag. 2014;4(3):271–82. https://doi.org/10.2217/nmt.14.17.
Martinez-Martin P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, Henriksen T, Martin A, Calandrella D, Rizos A, Bryndum N, Glad A, Dafsari HS, Timmermann L, Ebersbach G, Kramberger MG, Samuel M, Wenzel K, Tomantschger V, Storch A, Reichmann H, Pirtosek Z, Trost M, Svenningsson P, Palhagen S, Volkmann J, Chaudhuri KR. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510–6. https://doi.org/10.1002/mds.26067 (Epub 2014 Nov 10).
Drapier S, Eusebio A, Degos B, Vérin M, Durif F, Azulay JP, Viallet F, Rouaud T, Moreau C, Defebvre L, Fraix V, Tranchant C, Andre K, Courbon CB, Roze E, Devos D. Quality of life in Parkinson’s disease improved by apomorphine pump: the OPTIPUMP cohort study. J Neurol. 2016;263(6):1111–9. https://doi.org/10.1007/s00415-016-8106-3 (Epub 2016 Apr 8).
Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Lees A. Double-blind, randomized, placebo-controlled, Phase III study (TOLEDO) to evaluate the efficacy of apomorphine subcutaneous infusion in reducing OFF time in Parkinson’s disease patients with motor fluctuations not well controlled on optimized conventional treatment [abstract]. Mov Disord. 2017; 32 (suppl 2). http://www.mdsabstracts.org/abstract/double-blind-randomized-placebo-controlled-phase-iii-study-toledo-to-evaluate-the-efficacy-of-apomorphine-subcutaneous-infusion-in-reducing-off-time-in-parkinsons-disease-patients-with-m/ Accessed July 12, 2017.
Titova N, Chaudhuri KR. Apomorphine therapy in Parkinson’s and future directions. Parkinsonism Relat Disord. 2016;33(1):S56–60. https://doi.org/10.1016/j.parkreldis.2016.11.013 (Epub 2016 Nov 30).
Borkar N, Mu H, Holm R. Challenges trends in apomorphine drug demivery systems for the treatment of Parkinson’s disease. Asian J Pharmaceutical Sci. 2017. https://doi.org/10.1016/j.ajps.2017.11.004 (ISSN 1818–0876).
Titova N, Padmakumar C, Lewis SJG, Chaudhuri KR. Parkinson’s: a syndrome rather than a disease? J Neural Transm (Vienna). 2017;124(8):907–14. https://doi.org/10.1007/s00702-016-1667-6 (Epub 2016 Dec 27).
Brennan KA, Genever RW. Managing Parkinson’s disease during surgery. BMJ. 2010;341:c5718. https://doi.org/10.1136/bmj.c5718.
de Campos AM, Braz L, Linhares P, Rosas MJ. Deep brain stimulation for Parkinson’s disease: subcutaneous apomorphine as an alternative for patients unable to tolerate surgery under local anesthesia. J Neurol Sci. 2017;15(378):137–9. https://doi.org/10.1016/j.jns.2017.04.048 (Epub 2017 May 3).
Broussolle E, Marion MH, Pollak P. Continuous subcutaneous apomorphine as replacement for levodopa in severe parkinsonian patients after surgery. Lancet. 1992;340(8823):859–60.
Gálvez-Jiménez N, Lang AE. Perioperative problems in Parkinson’s disease and their management: apomorphine with rectal domperidone. Can J Neurol Sci. 1996;23(3):198–203.
Sharma JC, Macnamara L, Hasoon M, Vassallo M. Diagnostic and therapeutic value of apomorphine in Parkinsonian patients. Int J Clin Pract. 2004;58(11):1028–32.
Schlesinger I, Erikh I, Zaaroor M. Dopamine agonist withdrawal syndrome: the apomorphine solution. Arch Neurol. 2010;67(9):1155–6. https://doi.org/10.1001/archneurol.2010.220.
Slotty PJ, Wille C, Kinfe TM, Vesper J. Continuous perioperative apomorphine in deep brain stimulation surgery for Parkinson’s disease. Br J Neurosurg. 2014;28(3):378–82. https://doi.org/10.3109/02688697.2013.841859 (Epub 2013 Sep 27).
Dewhurst F, Lee M, Wood B. The pragmatic use of apomorphine at the end of life. Palliat Med. 2009;23(8):777–9. https://doi.org/10.1177/0269216309106979.
Bonuccelli U, Piccini P, Corsini GU, Muratorio A. Apomorphine in malignant syndrome due to levodopa withdrawal. Ital J Neurol Sci. 1992;13(2):169–70.
Rosa-Grilo M, Qamar MA, Evans A, Chaudhuri KR. The efficacy of apomorphine—a non-motor perspective. Parkinsonism Relat Disord. 2016;33(1):S28–35. https://doi.org/10.1016/j.parkreldis.2016.11.020.
Tison F, Wiart L, Guatterie M, Fouillet N, Lozano V, Henry P, Barat M. Effects of central dopaminergic stimulation by apomorphine on swallowing disorders in Parkinson’s disease. Mov Disord. 1996;11(6):729–32.
Hunter PC, Crameri J, Austin S, Woodward MC, Hughes AJ. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry. 1997;63(5):579–83.
Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Defecatory function in Parkinson’s disease: response to apomorphine. Ann Neurol. 1993;33(5):490–3.
Christmas TJ, Kempster PA, Chapple CR, Frankel JP, Lees AJ, Stern GM, Milroy EJ. Role of subcutaneous apomorphine in parkinsonian voiding dysfunction. Lancet. 1988;2(8626–8627):1451–3.
Bronner G, Vodušek DB. Management of sexual dysfunction in Parkinson’s disease. Ther Adv Neurol Disord. 2011;4(6):375–83. https://doi.org/10.1177/1756285611411504.
Fernández-Pajarín G, Sesar Á, Ares B, Castro A. Evaluating the efficacy of nocturnal continuous subcutaneous apomorphine infusion in sleep disorders in advanced parkinson’s disease: the APO-NIGHT study. J Parkinsons Dis. 2016;6(4):787–92.
Shaltiel-Karyo R, Tsarfati Y, Zawoznik E, Weinstock I, Nemas M, Rubinski A, Schiffenbauer YS, Nyska A, Yacoby-Zeevi O. ND0701: A novel safe concentrated apomorphine formulation for continuous subcutaneous administration via a patch pump (P4.006). Neurology. 2017;88:P4.006.
Borgemeester RW, Drent M, van Laar T. Motor and non-motor outcomes of continuous apomorphine infusion in 125 Parkinson’s disease patients. Parkinsonism Relat Disord. 2016;23:17–22. https://doi.org/10.1016/j.parkreldis.2015.11.013.
Borgemeester RW, Lees AJ, van Laar T. Parkinson’s disease, visual hallucinations and apomorphine: a review of the available evidence. Parkinsonism Relat Disord. 2016;27:35–40. https://doi.org/10.1016/j.parkreldis.2016.04.023.
Borgemeester RWK, van Laar T. Continuous subcutaneous apomorphine infusion in Parkinson’s disease patients with cognitive dysfunction: a retrospective long-term follow-up study. Parkinsonism Relat Disord. 2017;45:33–8. https://doi.org/10.1016/j.parkreldis.2017.09.025.
Geerligs L, Meppelink AM, Brouwer WH, van Laar T. The effects of apomorphine on visual perception in patients with Parkinson disease and visual hallucinations: a pilot study. Clin Neuropharmacol. 2009;32(5):266–8. https://doi.org/10.1097/wnf.0b013e3181a6a92b.
Yarnall AJ, Lashley T, Ling H, Lees AJ, Coleman SY, O’Sullivan SS, Compta Y, Revesz T, Burn DJ. Apomorphine: a potential modifier of amyloid deposition in Parkinson’s disease? Mov Disord. 2016;31(5):668–75. https://doi.org/10.1002/mds.26422 (Epub 2015 Oct 13).
Cousin MA, Ebbert JO, Wiinamaki AR, Urban MD, Argue DP, Ekker SC, Klee EW. Larval zebrafish model for FDA-approved drug repositioning for tobacco dependence treatment. PLoS ONE. 2014;9(3):e90467. https://doi.org/10.1371/journal.pone.0090467 (eCollection 2014).
Acknowledgements
Mrs. Elizabeth Portier-Wiles edited the manuscript for non-intellectual content.
Author information
Authors and Affiliations
Contributions
Dr. Manon Auffret: conception, organization and execution of the review project, manuscript preparation (writing of the first draft, review and critique). Dr. Sophie Drapier: manuscript review and critique. Prof. Marc Vérin: conception of the project, manuscript review and critique.
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosure
Dr. Manon Auffret: none. Dr. Sophie Drapier served on scientific advisory boards for Orkyn and Aguettant, received speech honorarium from Orkyn, Aguettant, Medtronic, and Teva, and received PHRC grants from the French Ministry of Health (unrelated to this article). Prof. Marc Vérin served on scientific advisory boards for Orkyn and Aguettant and received speech honorarium from Orkyn, Aguettant, Medtronic, and Teva (unrelated to this article).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Auffret, M., Drapier, S. & Vérin, M. The Many Faces of Apomorphine: Lessons from the Past and Challenges for the Future. Drugs R D 18, 91–107 (2018). https://doi.org/10.1007/s40268-018-0230-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40268-018-0230-3