Abstract
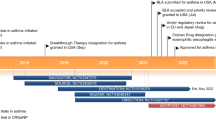
Thymic stromal lymphopoietin (TSLP) is an allarmin cytokine whose importance in human asthma has been repeatedly documented. Accordingly, targeting of TSLP and TSLP-mediated signalling is considered as an attractive therapeutic strategy to asthma. Tezepelumab, which is the first-in-class anti-TSLP monoclonal antibodies (mAb), is a fully human IgG2λ mAb that binds human TSLP, prevents interaction with its receptor and, consequently, inhibits multiple downstream inflammatory pathways. Because of the excellent results of Phase II trials, the Food and Drug Administration granted tezepelumab as a ‘breakthrough’ biological drug for the treatment of severe asthma. Several studies with this mAb are ongoing. CSJ117 is an Ab fragment that binds to TSLP and is delivered by inhalation but there is no published information on this biologic agent. Since new information suggests that targeting TSLP may be more likely to improve day-to-day asthma symptoms, in contrast to targeting mediators of the adaptive immune system, approaches that primarily act to ameliorate asthma exacerbations, novel approaches capable of blocking TSLP (for example, fully human single-chain fragment variables against TSLP, bifunctional drugs such as the one that combines an anti-IL-13 mAb with an anti-TSLP mAb, a fusion protein consisting of the ectodomains of TSLPR and IL-7Ra that extend into the extracellular space, also known as a TSLP-trap, fragments capable of disrupting the TSLP:TSLPR complex) are under preclinical investigation. However, some critical aspects remain to be clarified before being able to define this approach as the one that will probably better help patients suffering from severe asthma because of its holistic effects.

Similar content being viewed by others
References
Mitchell PD, O’Byrne PM. Biologics and the lung: TSLP and other epithelial cell-derived cytokines in asthma. Pharmacol Ther. 2017;169:104–12.
Verstraete K, Peelman F, Braun H, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun. 2017;8:14937.
Semlali A, Jacques E, Koussih L, et al. Thymic stromal lymphopoietin-induced human asthmatic airway epithelial cell proliferation through an IL-13-dependent pathway. J Allergy Clin Immunol. 2010;125(4):844–50.
Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130(4):845–52.
Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med. 2013;19(8):1005–13.
Redhu NS, Gounni AS. Function and mechanisms of TSLP/TSLPR complex in asthma and COPD. Clin Exp Allergy. 2012;42(7):994–1005.
Datta A, Alexander R, Sulikowski MG, et al. Evidence for a functional thymic stromal lymphopoietin signalling axis in fibrotic lung disease. J Immunol. 2013;191(9):4867–79.
Comeau MR, Ziegler SF. The influence of TSLP on the allergic response. Mucosal Immunol. 2010;3(2):138–47.
Brandelius A, Yudina Y, Calvén J, et al. dsRNA-induced expression of thymic stromal lymphopoietin (TSLP) in asthmatic epithelial cells is inhibited by a small airway relaxant. Pulm Pharmacol Ther. 2011;24(1):59–66.
Tsilingiri K, Fornasa G, Rescigno M. Thymic stromal lymphopoietin: to cut a long story short. Cell Mol Gastroenterol Hepatol. 2017;3(2):174–82.
Park JH, Jeong DY, Peyrin-Biroulet L, et al. Insight into the role of TSLP in inflammatory bowel diseases. Autoimmun Rev. 2017;16(1):55–63.
Xie Y, Takai T, Chen X, et al. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci. 2012;66(3):233–7.
Dong H, Hu Y, Liu L, et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep. 2016;6:39559.
Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. 2018;9:1595.
Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signalling. Proc Natl Acad Sci USA. 2010;107(45):19455–60.
Yu X, Li H, Ren X. Signalling cascades initiated by TSLP-mediated signals in different cell types. Cell Immunol. 2012;279(2):174–9.
Borowski A, Vetter T, Kuepper M, et al. Expression analysis and specific blockade of the receptor for human thymic stromal lymphopoietin (TSLP) by novel antibodies to the human TSLPRα receptor chain. Cytokine. 2013;61(2):546–55.
Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–23.
So T, Song J, Sugie K, et al. Signals from OX40 regulate nuclear factor of activated T cells c1 and T cell helper 2 lineage commitment. Proc Natl Acad Sci USA. 2006;103(10):3740–5.
Dorman SC, Efthimiadis A, Babirad I, et al. Sputum CD34+IL-5Rα+ cells increase after allergen: evidence for in situ eosinophilopoiesis. Am J Respir Crit Care Med. 2004;169(5):573–7.
Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477(7363):229–33.
Hui CC, Rusta-Sallehy S, Asher I, et al. The effects of thymic stromal lymphopoietin and IL-3 on human eosinophil-basophil lineage commitment: relevance to atopic sensitization. Immun Inflamm Dis. 2014;2(1):44–55.
Smith SG, Gugilla A, Mukherjee M, et al. Thymic stromal lymphopoietin and IL-33 modulate migration of hematopoietic progenitor cells in patients with allergic asthma. J Allergy Clin Immunol. 2015;135(6):1594–602.
Kouzaki H, O’Grady SM, Lawrence CB, et al. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183(2):1427–34.
Salter BMA, Smith SG, Mukherjee M, et al. Human bronchial epithelial cell-derived factors from severe asthmatic subjects stimulate eosinophil differentiation. Am J Respir Cell Mol Biol. 2018;58(1):99–106.
Headley MB, Zhou B, Shih WX, et al. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182(3):1641–7.
Shi L, Leu SW, Xu F, et al. Local blockade of TSLP receptor alleviated allergic disease by regulating airway dendritic cells. Clin Immunol. 2008;129(2):202–10.
Chen ZG, Zhang TT, Li HT, et al. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One. 2013;8(1):e51268.
Salter BM, Oliveria JP, Nusca G, et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol. 2015;136(6):1636–44.
Wang W, Li Y, Lv Z, et al. Bronchial allergen challenge of patients with atopic asthma triggers an allarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J Immunol. 2018;201(8):2221–31.
Ying S, O’Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181(4):2790–8.
Ying S, O’Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174(12):8183–90.
Shikotra A, Choy DF, Ohri CM, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129(1):104–11.
Liu S, Verma M, Michalec L, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141(1):257–68.
Park S, Park Y, Son SH, et al. Synthesis and biological evaluation of peptide-derived TSLP inhibitors. Bioorg Med Chem Lett. 2017;27(20):4710–3.
Zhang F, Huang G, Hu B, et al. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol. 2011;164(2):256–64.
Fuentes M, Ma X, Zhang J, et al. Anti-TSLPR antibody RG7258 blocks dendritic cell activation, mast cell cytokine release and reduces Th2 inflammation in a non-human primate model of allergic lung inflammation [abstract]. Am J Respir Crit Care Med. 2011;183:A2767.
Numazaki M, Hanaoka K, Imamura E, et al. ASP7266, a novel antibody against human TSLPR, in the treatment of allergic disease [abstract]. J Allergy Clin Immunol. 2018;141(2):AB13.
Parnes JR, Sullivan JT, Chen L, et al. Pharmacokinetics, safety, and tolerability of tezepelumab (AMG 157) in healthy and atopic dermatitis adult Subjects. Clin Pharmacol Ther. 2019;106(2):441–9.
Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–10.
Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–46.
Pham T-H, Ren P, Parnes JR, et al. Tezepelumab reduces multiple key inflammatory biomarkers in patients with severe, uncontrolled asthma in the phase 2b PATHWAY study [abstract]. Am J Respir Crit Care Med. 2019;199:A2677.
Corren J, Chen S, Callan L, et al. The impact of tezepelumab on hospitalization and emergency department visits in patients with severe uncontrolled asthma: results from the pathway phase 2b trial [abstract]. Am J Respir Crit Care Med. 2019;199:A2622.
Ly N, Zheng Y, Griffiths JM, et al. Exposure-response analysis of tezepelumab in patients with severe asthma to guide phase 3 dose selection [abstract]. Eur Respir J. 2018;52(suppl 62):PA1688.
FDANews. FDA awards AstraZeneca and Amgen’s tezepelumab breakthrough designation. https://www.fdanews.com/articles/188355-fda-awards-astrazeneca-and-amgens-tezepelumab-breakthrough-designation. Accessed 10 Nov 2019.
Edris A, De Feyter S, Maes T, et al. Monoclonal antibodies in type 2 asthma: a systematic review and network meta-analysis. Respir Res. 2019;20(1):179.
Nian S, Zhu J, Yu H, et al. Development and identification of a fully human single-chain variable fragment 29 against TSLP. Biotechnol Appl Biochem. 2019;66(4):510–6.
Page C, Cazzola M. Bifunctional drugs for the treatment of respiratory diseases. Handb Exp Pharmacol. 2017;237:197–212.
Venkataramani S, Low S, Weigle B, et al. Design and characterization of Zweimab and Doppelmab, high affinity dual antagonistic anti-TSLP/IL13 bispecific antibodies. Biochem Biophys Res Commun. 2018;504(1):19–24.
Economides AN, Carpenter LR, Rudge JS, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9(1):47–52.
Van Rompaey D, Verstraete K, Peelman F, et al. Virtual screening for inhibitors of the human TSLP:TSLPR interaction. Sci Rep. 2017;7(1):17211.
Park BB, Choi JW, Park D, et al. Structure-activity relationships of baicalein and its analogs as novel TSLP inhibitors. Sci Rep. 2019;9(1):8762.
Moon PD, Han NR, Ryu KJ, et al. A novel compound 2-(4- {2-[(phenylthio)acetyl]carbonohydrazonoyl}phenoxy)acetamide downregulates TSLP through blocking of caspase-1/NF-jB pathways. Int Immunopharmacol. 2016;38:420–5.
Moon PD, Han NR, Lee JS, et al. Effects of linalyl acetate on thymic stromal lymphopoietin production in mast cells. Molecules. 2018;23(7):E1711.
Pan Z, Zhou Y, Luo X, et al. Against NF-κB/thymic stromal lymphopoietin signalling pathway, catechin alleviates the inflammation in allergic rhinitis. Int Immunopharmacol. 2018;61:241–8.
Segawa R, Shiraki M, Sudo S, et al. A chalcone derivative suppresses the induction of TSLP in mice and human keratinocytes and attenuates OVA-induced antibody production in mice. Eur J Pharmacol. 2019;851:52–62.
Borish L. The immunology of asthma: asthma phenotypes and their implications for personalized treatment. Ann Allergy Asthma Immunol. 2016;117(2):108–14.
Marone G, Spadaro G, Braile M, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 2019;28(11):931–40.
Ohnishi H, Yokoyama A. Future treatment and other new biologics for asthma. In: Yokoyama A, editor. Advances in asthma, respiratory disease series: diagnostic tools and disease managements. Singapore: Springer Nature Singapore Pte Ltd; 2019. p. 177–89.
An G, Wang W, Zhang X, et al. Combined blockade of IL-25, IL-33 and TSLP mediates amplified inhibition of airway inflammation and remodelling in a murine model of asthma. Respirology. 2019. https://doi.org/10.1111/resp.13711.
Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22(4):494–505.
Di Piazza M, Nowell CS, Koch U, et al. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell. 2012;22(4):479–93.
Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat Immunol. 2019;20(12):1603–9.
Orellana A, García-González V, López R, et al. Application of a phenotypic drug discovery strategy to identify biological and chemical starting points for inhibition of TSLP production in lung epithelial cells. PLoS One. 2018;13(1):e0189247.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mario Cazzola, Paola Rogliani, Luigino Calzetta and Maria Gabriella Matera have no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript, including employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Funding
This manuscript was not funded/sponsored, and no writing assistance was utilized in its production.
Rights and permissions
About this article
Cite this article
Matera, M.G., Rogliani, P., Calzetta, L. et al. TSLP Inhibitors for Asthma: Current Status and Future Prospects. Drugs 80, 449–458 (2020). https://doi.org/10.1007/s40265-020-01273-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01273-4