Abstract
Background and Objective
Mildronate, an inhibitor of carnitine-dependent metabolism, is considered to be an anti-ischemic drug. This study is designed to evaluate the efficacy and safety of mildronate injection in treating acute ischemic stroke.
Methods
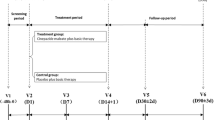
We performed a randomized, double-blind, multicenter clinical study of mildronate injection for treating acute cerebral infarction. 113 patients in the experimental group received mildronate injection, and 114 patients in the active-control group received cinepazide injection. In addition, both groups were given aspirin as a basic treatment. Modified Rankin Scale (mRS) score was performed at 2 weeks and 3 months after treatment. National Institutes of Health Stroke Scale (NIHSS) score and Barthel Index (BI) score were performed at 2 weeks after treatment, and then vital signs and adverse events were evaluated.
Results
A total of 227 patients were randomized to treatment (n = 113, mildronate; n = 114, active-control). After 3 months, there was no significant difference for the primary endpoint between groups categorized in terms of mRS scores of 0–1 and 0–2 (p = 0.52 and p = 0.07, respectively). There were also no significant differences for the secondary endpoint between groups categorized in terms of NIHSS scores of >5 and >8 (p = 0.98 and p = 0.97, respectively) or BI scores of >75 and >95 (p = 0.49 and p = 0.47, respectively) at 15 days. The incidence of serious adverse events was similar between the two groups.
Conclusion
Mildronate injection is as effective and safe as cinepazide injection in treating acute cerebral infarction.

Similar content being viewed by others
References
Naylor AR. Letter by Naylor regarding article, “Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association”. Stroke. 2011;42(6):e385; author reply e386.
Wood H. Stroke: could the neuroprotective drug NA-1 limit ischaemic brain damage after stroke? Nat Rev Neurol. 2012;8(12):658.
Blanco M, Castillo J. Stroke in 2012: major advances in the treatment of stroke. Nat Rev Neurol. 2013;9(2):68–70.
Kingwell K. Stroke: neuroprotection for patients with stroke moves one step closer to the clinic. Nat Rev Neurol. 2012;8(4):178.
Cook DJ, Teves L, Tymianski M. Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic primate brain. Nature. 2012;483(7388):213–7.
Bach A, Clausen BH, Moller M, et al. A high-affinity, dimeric inhibitor of PSD-95 bivalently interacts with PDZ1-2 and protects against ischemic brain damage. Proc Natl Acad Sci USA. 2012;109(9):3317–22.
Vilskersts R, Liepinsh E, Mateuszuk L, et al. Mildronate, a regulator of energy metabolism, reduces atherosclerosis in apoE/LDLR−/− mice. Pharmacology. 2009;83(5):287–93.
Okunevich IV, Ryzhenkov VE. Anti-atherosclerotic action of mildronate in experiment [in Russian]. Patol Fiziol Eksp Ter. 2002;2:24–7.
Dambrova M, Liepinsh E, Kalvinsh I. Mildronate: cardioprotective action through carnitine-lowering effect. Trends Cardiovasc Med. 2002;12(6):275–9.
Liepinsh E, Vilskersts R, Loca D, et al. Mildronate, an inhibitor of carnitine biosynthesis, induces an increase in gamma-butyrobetaine contents and cardioprotection in isolated rat heart infarction. J Cardiovasc Pharmacol. 2006;48(6):314–9.
Vilskersts R, Liepinsh E, Kuka J, et al. Myocardial infarct size-limiting and anti-arrhythmic effects of mildronate orotate in the rat heart. Cardiovasc Drugs Ther. 2009;23(4):281–8.
Statsenko ME, Poletaeva LV, Turkina SV, et al. Mildronate effects on oxidant stress in type 2 diabetic patients with diabetic peripheral (sensomotor) neuropathy [in Russian]. Ter Arkh. 2008;80(10):27–30.
Sokolovska J, Rumaks J, Karajeva N, et al. The influence of mildronate on peripheral neuropathy and some characteristics of glucose and lipid metabolism in rat streptozotocin-induced diabetes mellitus model [in Russian]. Biomed Khim. 2011;57(5):490–500.
Vilskersts R, Kuka J, Svalbe B, et al. Administration of l-carnitine and mildronate improves endothelial function and decreases mortality in hypertensive Dahl rats. Pharmacol Rep. 2011;63(3):752–62.
Beketov AI, Mametova AN, Polevik IV, et al. Comparative characteristics of cerebrovascular protective effects of mildronate, riboxine, and their combination during modeling of cerebral hemodynamics disturbance [in Russian]. Eksp Klin Farmakol. 2000;63(6):18–21.
Dziak LA, Golik VA. Use of mildronate for the treatment of patients with circulatory encephalopathy against a background of stenosis of major arteries of the head [in Russian]. Lik Sprava. 2003;5–6:98–101.
Sjakste N, Baumane L, Boucher JL, et al. Effects of gamma-butyrobetaine and mildronate on nitric oxide production in lipopolysaccharide-treated rats. Basic Clin Pharmacol Toxicol. 2004;94(1):46–50.
Sjakste N, Kleschyov AL, Boucher JL, et al. Endothelium- and nitric oxide-dependent vasorelaxing activities of gamma-butyrobetaine esters: possible link to the antiischemic activities of mildronate. Eur J Pharmacol. 2004;495(1):67–73.
Liepinsh E, Konrade I, Skapare E, et al. Mildronate treatment alters gamma-butyrobetaine and l-carnitine concentrations in healthy volunteers. J Pharm Pharmacol. 2011;63(9):1195–201.
Svalbe B, Zvejniece L, Vavers E, et al. Mildronate treatment improves functional recovery following middle cerebral artery occlusion in rats. Behav Brain Res. 2011;222(1):26–32.
Pupure J, Isajevs S, Skapare E, et al. Neuroprotective properties of mildronate, a mitochondria-targeted small molecule. Neurosci Lett. 2010;470(2):100–5.
Isajevs S, Isajeva D, Beitnere U, et al. Mildronate as a regulator of protein expression in a rat model of Parkinson’s disease. Medicina (Kaunas). 2011;47(10):552–9.
Sjakste N, Gutcaits A, Kalvinsh I. Mildronate: an antiischemic drug for neurological indications. CNS Drug Rev. 2005;11(2):151–68.
Fearon P, McArthur KS, Garrity K, et al. Prestroke modified Rankin Stroke Scale has moderate interobserver reliability and validity in an acute stroke setting. Stroke. 2012;43(12):3184–8.
Liu X, Xia J, Wang L, et al. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol. 2009;16(5):569–75.
Liu X, Wang L, Wen A, et al. Ginsenoside-Rd improves outcome of acute ischaemic stroke - a randomized, double-blind, placebo-controlled, multicenter trial. Eur J Neurol. 2012;19(6):855–63.
Akashi A, Hirohashi M, Suzuki I, et al. Cardiovascular pharmacology of cinepazide, a new cerebral vasodilator (author’s transl) [in Japanese]. Nihon Yakurigaku Zasshi. 1979;75(5):507–16.
Baba M, Kitamura K. The effects of cinepazide on cerebral circulation (author’s transl) [in Japanese]. No To Shinkei. 1979;31(6):621–9.
Warembourg G, Carre A, Ginestet A, et al. Clinical experimentation with a new vasodilator: cinepazide maleate in arterial diseases of the lower limbs [in French]. Lille Med. 1976;21(Suppl 4):898–901.
Moritake K, Handa H, Takebe Y, et al. Effect of intravenous administration of Cinepazide on cerebral blood flow and evoked potentials [in Japanese]. Nihon Geka Hokan. 1983;52(2):207–17.
Kitaoka H, Ohya K, Sano M. Interaction between cinepazide maleate, a new cerebral vasodilator, and water [in Japanese]. Yakugaku Zasshi. 1983;103(1):28–33.
Fujishima M. Agents to improve cerebrovascular circulation and cerebral metabolism–cinepazide [in Japanese]. Nihon Rinsho. 1985;43(2):379-82.
Muramatsu I, Sakakibara Y, Hong SC, et al. Effects of cinepazide on the purinergic responses in the dog cerebral artery. Pharmacology. 1984;28(1):27–33.
Sesti C, Simkhovich BZ, Kalvinsh I, et al. Mildronate, a novel fatty acid oxidation inhibitor and antianginal agent, reduces myocardial infarct size without affecting hemodynamics. J Cardiovasc Pharmacol. 2006;47(3):493–9.
Stanley WC. Partial fatty acid oxidation inhibitors for stable angina. Expert Opin Investig Drugs. 2002;11(5):615–29.
Dambrova M, Daiia D, Liepin’Sh E, et al. Biochemical mechanisms of mildronate action during ischemic stress [in Russian]. Lik Sprava. 2004;2:68–74.
Zvejniece L, Svalbe B, Makrecka M, et al. Mildronate exerts acute anticonvulsant and antihypnotic effects. Behav Pharmacol. 2010;21(5–6):548–55.
Liepinsh E, Vilskersts R, Skapare E, et al. Mildronate decreases carnitine availability and up-regulates glucose uptake and related gene expression in the mouse heart. Life Sci. 2008;83(17–18):613–9.
Sjakste N, Gutcaits A, Kalvinsh I. Mildronate: an antiischemic drug for neurological indications. CNS Drug Rev. 2005;11(2):151–68.
Acknowledgments
This study was funded by Ge-Lin Biopharmaceutical Co. Ltd., Shenyang, China. The authors have no potential conflicts of interest that are directly relevant to the content of this study. The Steering Committee for this study consisted of Prof. Su Xiuchu, Prof. Xu Dezhong, Prof. Wu Baoren, and Dr. Fan Fulin. The Safety Board consisted of Prof. Huang Yuangui, Prof. Li Huanzhang, and Prof. Li Kaizong.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Zhu and G. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, Y., Zhang, G., Zhao, J. et al. Efficacy and Safety of Mildronate for Acute Ischemic Stroke: A Randomized, Double-Blind, Active-Controlled Phase II Multicenter Trial. Clin Drug Investig 33, 755–760 (2013). https://doi.org/10.1007/s40261-013-0121-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-013-0121-x