Abstract
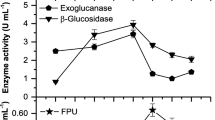
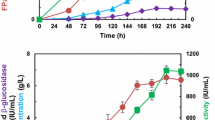
In this study, extracellular enzyme production by different species of Trichoderma fungus (T. aureoviride NAS106, T. afroharzianum NAS107, T. ghanense NAS108, T. pleuroticola NAS109, T. harzianum NAS110, and T. lixii NAS114) were investigated under submerged fermentation conditions using lemon peel waste as a substrate at 28 °C, pH 5, and fermentation time of 48 h. Extracellular protein concentration, cellulose (exoglucanase, endoglucanase, β-glucosidase, and total cellulase), xylanase, and pectinase enzyme activity were also assayed. This study also examined the effect of different fermentation times on extracellular protein production and enzymatic activity in a superior strain of Trichoderma. The molecular weight of extracellular proteins was studied using SDS-PAGE. The results showed that the highest extracellular protein concentration and exoglucanase, endoglucanase, beta-glucosidase, total cellulase, xylanase, and pectinase activity were produced in T. afroharzianum NAS107. It was also revealed that the major activity of the total cellulase enzyme was due to the synergistic function of endoglucanases and cellulohydrolases. In addition, the electrophoretic pattern of protein bands of this species showed the ability to produce enzymes of xylanase (Xyl I), exoglucanase (Cel 6A (CBH II) and Cel7A (CBH I)), endoglucanases (Cel 5A (EG II), and Cel 12A (EG III), β-glucosidase (Cel3A (BGL I) and Cel 1A (BGL II)), polygalacturonase I and II, pectin lyase, and pectin esterase I and II, with the highest enzymatic activity during the fermentation period of 96 to 120 h due to the synergistic activity of exoglucanase, endoglucanase, beta-glucosidase, and xylanase enzymes. The findings of this study showed that T. afroharzianum is a suitable producer of extracellular enzymes cellulase, xylanase, and pectinase. Thus, it can be used as a biological substance or an enzyme source for saccharification of citrus conversion industry wastes such as lemon peel.




Similar content being viewed by others
References
Bilal M, Iqbal HM, Hu H, Wang W, Zhang X (2018) Metabolic engineering and enzyme-mediated processing: a biotechnological venture towards biofuel production–a review. Renew Sustain Energy Rev 82:436–447. https://doi.org/10.1016/j.rser.2017.09.070
FN Niyonzima 2021 Detergent-compatible fungal cellulases Folia microbiologica 66 25 40 https://doi.org/10.1007/s12223-020-00838-w
Buenrostro-Figueroa J, de la Garza-Toledo H, Ibarra-Junquera V, Aguilar CN (2010) Juice extraction from mango pulp using an enzymatic complex of Trichoderma sp. produced by solid-state fermentation. Food Sci Biotechnol 19:1387–1390. https://doi.org/10.1007/s10068-010-0197-5
Sun J, Lu J, Xie G (2018) Secretome analysis of Trichoderma reesei CICC41495 for degradation of arabinoxylan in malted barley. J Inst Brew 124:352–358. https://doi.org/10.1002/jib.505
Verma N, Kumar V, Bansal MC (2018) Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal Agric Biotechnol 16:483–492. https://doi.org/10.1016/j.bcab.2018.09.021
Al-Maqtari QA, Waleed A-A, Mahdi AA (2019) Microbial enzymes produced by fermentation and their applications in the food industry-a review. Int J Agric Innov Res 8:62–82
Carvalho EA, dos Santos Góes LM, Uetanabaro APT, da Silva EGP, Rodrigues LB, Pirovani CP, da Costa AM (2017) Thermoresistant xylanases from Trichoderma stromaticum: application in bread making and manufacturing xylo-oligosaccharides. Food Chem 221:1499–1506. https://doi.org/10.1016/j.foodchem.2016.10.144
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87:787–799. https://doi.org/10.1007/s00253-010-2632-1
AC Costa da GF Cavalheiro ER Queiroz Vieira de JR Gandra e BuschinelliRHdT, da Paz MF, Fonseca GG, Leite RSR, 2019 Catalytic properties of xylanases produced by Trichoderma piluliferum and Trichoderma viride and their application as additives in bovine feeding Biocatal Agric Biotechnol 19 101161 https://doi.org/10.1016/j.bcab.2019.101161
N Srivastava M Srivastava A Alhazmi T Kausar S Haque R Singh PW Ramteke PK Mishra M Tuohy M Leitgeb 2021 Technological advances for improving fungal cellulase production from fruit wastes for bioenergy application: a review Environ Pollut 117370 https://doi.org/10.1016/j.envpol.2021.117370
Arora N, Banerjee AK, Mutyala S, Murty US (2009) Comparative characterization of commercially important xylanase enzymes. Bioinformation 3:446. https://doi.org/10.6026/97320630003446
Prasanna H, Ramanjaneyulu G, Reddy BR (2016) Optimization of cellulase production by Penicillium sp. 3 Biotech 6: 1–11. https://doi.org/10.1007/s13205-016-0483-x
Bansal N, Tewari R, Soni R, Soni SK (2012) Production of cellulases from Aspergillus niger NS-2 in solid state fermentation on agricultural and kitchen waste residues. Waste Manage 32:1341–1346. https://doi.org/10.1016/j.wasman.2012.03.006
Liu J, Yang J, Wang R, Liu L, Zhang Y, Bao H, Jang JM, Wang E, Yuan H (2020) Comparative characterization of extracellular enzymes secreted by Phanerochaete chrysosporium during solid-state and submerged fermentation. Int J Biol Macromol 152:288–294. https://doi.org/10.1016/j.ijbiomac.2020.02.256
Kunamneni A, Plou FJ, Alcalde M, Ballesteros A (2014) Trichoderma enzymes for food industries. in: Biotechnology and biology of Trichoderma. Elsevier, pp 339–344. https://doi.org/10.1016/B978-0-444-59576-8.00024-2
Burkert J, Maugeri F, Rodrigues M (2004) Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Biores Technol 91:77–84. https://doi.org/10.1016/S0960-8524(03)00152-4
N Verma V Kumar 2020 Utilization of bottle gourd vegetable peel waste biomass in cellulase production by Trichoderma reesei and Neurosporacrassa Biomass Convers Biorefine 1–10 https://doi.org/10.1007/s13399-020-00727-9
Zehra M, Syed MN, Sohail M (2020) Banana peels: a promising substrate for the coproduction of pectinase and xylanase from Aspergillus fumigatus MS16. Polish journal of microbiology 69: 19–26. https://doi.org/10.33073/pjm-2020-002
Irfan M, Bakhtawar J, Sadia S, Shakir HA, Khan M, Ali S (2020) Utilization of fruit wastes for enzyme production in submerged fermentation. International Journal of Biology and Chemistry 13: 88–95. https://doi.org/10.26577/ijbch.2020.v13.i2.11
Ruiz HA, Rodríguez-Jasso RM, Rodríguez R, Contreras-Esquivel JC, Aguilar CN (2012) Pectinase production from lemon peel pomace as support and carbon source in solid-state fermentation column-tray bioreactor. Biochem Eng J 65:90–95. https://doi.org/10.1016/j.bej.2012.03.007
Mukherjee R, Paul T, Soren JP, Halder SK, Mondal KC, Pati BR, Mohapatra PKD (2019) Acidophilic α-amylase production from Aspergillus niger RBP7 using potato peel as substrate: a waste to value added approach. Waste biomass valoriz 10:851–863. https://doi.org/10.1007/s12649-017-0114-8
Mamma D, Kourtoglou E, Christakopoulos P (2008) Fungal multienzyme production on industrial by-products of the citrus-processing industry. Biores Technol 99:2373–2383. https://doi.org/10.1016/j.biortech.2007.05.018
Li P-J, Xia J-L, Shan Y, Nie Z-Y, Su D-L, Gao Q-R, Zhang C, Ma Y-L (2015) Optimizing production of pectinase from orange peel by Penicillium oxalicum PJ02 using response surface methodology. Waste Biomass Valoriz 6:13–22. https://doi.org/10.1007/s12649-014-9317-4
FAO (2019) Food and Agriculture Organization Citrus production data. http://www.fao.org. 10 Aug 2021
Al-Qudah TS, Zahra U, Rehman R, Majeed MI, Sadique S, Nisar S, Tahtamouni R, Tahtamouni RW (2018) Lemon as a source of functional and medicinal ingredient: a review. Int J Chem Biochem Sci 14:55–61
Sharma H, Singh I, Misra JP (2019) Mechanical and thermal behaviour of food waste (Citrus limetta peel) fillers–based novel epoxy composites. Polym Polym Compos 27:527–535. https://doi.org/10.1177/0967391119851012
Gómez-Mejía E, Rosales-Conrado N, León-González ME, Madrid Y (2019) Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem 295:289–299. https://doi.org/10.1016/j.foodchem.2019.05.136
Garcia-Castello EM, Rodriguez-Lopez AD, Mayor L, Ballesteros R, Conidi C, Cassano A (2015) Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci Technol 64:1114–1122. https://doi.org/10.1016/j.lwt.2015.07.024
Bagde PP, Dhenge S, Bhivgade S (2017) Extraction of pectin from orange peel and lemon peel. Int J Eng Technol Sci Res 4:1–7
AOAC (2000) Official methods of analysis Seventeenth edn. Association of Official Analytical Chemists, Gaithersburg
Shahbazi S, Askari H (2018) Investigating of the influence of cellulase enzymes from mutated isolates of Trichoderma harzianum and T. viride on biodegradation of cellulose Iα, Iβ and III. Agric Biotechnol https://doi.org/10.22084/AB.2018.7877.1249
Shahbazi S, Askari H, Mojerlou S (2016) The impact of different physicochemical parameters of fermentation on extracellular cellulolytic enzyme production by Trichoderma harzianum. J Crop Protect 5: 397–412. https://doi.org/10.18869/modares.jcp.5.3.397
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Gama F, Mota M (1998) Cellulases for oligosaccharide synthesis: a preliminary study. Carbohyd Polym 37:279–281. https://doi.org/10.1016/S0144-8617(98)00071-X
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature 227: 680–685. https://doi.org/10.1038/227680a0
Chinedu SN, Okochi V, Omidiji O (2011) Cellulase production by wild strains of Aspergillus niger, Penicillium chrysogenum and Trichoderma harzianum grown on waste cellulosic materials. IFE J Sci 13:57–62
Durand H, Clanet M, Tiraby G (1988) Genetic improvement of Trichoderma reesei for large scale cellulase production. Enzyme Microb Technol 10:341–346. https://doi.org/10.1016/0141-0229(88)90012-9
Askari H, Shahbazi S (2018) Improvement of cellulose degrading enzymes activity by mutagenesis in Trichoderma reesei fungi. Agricultural Biotechnology 9:41–50
Shahbazi S, Askari H, Ebrahimi A, Safaeie M, Karimi M (2015) Increasing the efficiency of sugar beet pulp saccharification by Trichoderma reesei superior mutants for bioethanol production. Journal of Sugar Beet 31 :61–76. https://doi.org/10.22092/JSB.2015.101498.
Wood TM, Bhat KM (1988) Methods for measuring cellulase activities. Methods Enzymol 160:87–112. https://doi.org/10.1016/0076-6879(88)60109-1
van Tilbeurgh H, Claeyssens M, de Bruyne CK (1982) The use of 4-methylumbelliferyl and other chromophoric glycosides in the study of cellulolytic enzymes. FEBS Lett 149:152–156. https://doi.org/10.1016/0014-5793(82)81092-2
H Tilbeurgh van G Pettersson R Bhikabhai H Boeck de CLAEYSSENS M, 1985 Studies of the cellulolytic system of Trichoderma reesei QM 9414: reaction specificity and thermodynamics of interactions of small substrates and ligands with the 1, 4-β-glucan cellobiohydrolase II Eur J Biochem 148 329 334 https://doi.org/10.1111/j.1432-1033.1985.tb08843.x
Väljamäe P, Sild V, Pettersson G, Johansson G (1998) The initial kinetics of hydrolysis by cellobiohydrolases I and II is consistent with a cellulose surface− erosion model. Eur J Biochem 253:469–475. https://doi.org/10.1046/j.1432-1327.1998.2530469.x
Srisodsuk M, Kleman-Leyer K, Keränen S, Kirk TK, Teeri TT (1998) Modes of action on cotton and bacterial cellulose of a homologous endoglucanase-exoglucanase pair from Trichoderma reesei. Eur J Biochem 251:885–892. https://doi.org/10.1046/j.1432-1327.1998.2510885.x
Nidetzky B, Claeyssens M (1994) Specific quantification of Trichoderma reesei cellulases in reconstituted mixtures and its application to cellulase–cellulose binding studies. Biotechnol Bioeng 44:961–966. https://doi.org/10.1002/bit.260440812
Medve J, Ståhlberg J, Tjerneld F (1994) Adsorption and synergism of cellobiohydrolase I and II of Trichoderma reesei during hydrolysis of microcrystalline cellulose. Biotechnol Bioeng 44:1064–1073. https://doi.org/10.1002/bit.260440907
Klyosov AA (1990) Trends in biochemistry and enzymology of cellulose degradation. Biochemistry 29:10577–10585. https://doi.org/10.1021/bi00499a001
Shoemaker S, Brown Jr R (1978) Characterization of endo-1, 4-β-d-glucanases purified from Trichoderma viride. Biochimica et Biophysica Acta (BBA)-Enzymology 523: 147–161. https://doi.org/10.1016/0005-2744(78)90017-7
Zhang YHP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng 88:797–824. https://doi.org/10.1002/bit.20282
Teeri T, Koivula A (1995) Cellulose degradation by native and engineered fungal cellulases. Carbohydr Eur 12:28–33
Zhang S, Wolfgang DE, Wilson DB (1999) Substrate heterogeneity causes the nonlinear kinetics of insoluble cellulose hydrolysis. Biotechnol Bioeng 66:35–41. https://doi.org/10.1002/(SICI)1097-0290(1999)66:1
Zhang YHP, Lynd LR (2006) A functionally based model for hydrolysis of cellulose by fungal cellulase. Biotechnol Bioeng 94:888–898. https://doi.org/10.1002/bit.20906
Fortkamp D, Knob A (2014) High xylanase production by Trichoderma viride using pineapple peel as substrate and its apllication in pulp biobleaching. Afr J Biotech 13:2248–2259. https://doi.org/10.5897/AJB2013.13479
Lappalainen A, Siika-Aho M, Kalkkinen N, Fagerström R, Tenkanen M (2000) Endoxylanase II from Trichoderma reesei has several isoforms with different isoelectric points. Biotechnol Appl Biochem 31:61–68. https://doi.org/10.1042/BA19990066
Stålbrand H, Siika-aho M, Tenkanen M, Viikari L (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29:229–242. https://doi.org/10.1016/0168-1656(93)90055-R
Mohamed SA, Al-Malki AL, Kumosani TA (2009) Characterization of a polygalacturonase from Trichoderma harzianum grown on citrus peel with application for apple juice. Aust J Basic Appl Sci 3:2770–2777
T Kobayashi K Koike T Yoshimatsu N HigakiSUzUMATSU A, OzAwA T, Hatada Y, Ito S, 1999 Purification and properties of a low-molecular-weight, high-alkaline pectate lyase from an alkaliphilic strain of Bacillus Biosci Biotechnol Biochem 63 65 72 https://doi.org/10.1271/bbb.63.65
Semenova M, Grishutin S, Gusakov A, Okunev O, Sinitsyn A (2003) Isolation and properties of pectinases from the fungus Aspergillus japonicus. Biochem Mosc 68:559–569. https://doi.org/10.1023/A:1023959727067
LD Kluskens ALEBEEK G-JWv, Voragen AG, VOS WMd, OOST Jvd, 2003 Molecular and biochemical characterization of the thermoactive family 1 pectate lyase from the hyperthermophilic bacterium Thermotoga maritima Biochem J 370 651 659 https://doi.org/10.1042/bj20021595
Acknowledgements
The authors gratefully acknowledge the Agricultural Sciences and Natural Resources University of Khuzestan for supporting of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gooruee, R., Hojjati, M., Behbahani, B.A. et al. Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Conv. Bioref. 14, 2777–2786 (2024). https://doi.org/10.1007/s13399-022-02626-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02626-7