Abstract
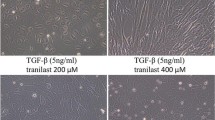
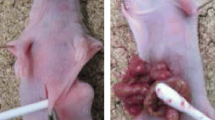
Peritoneal dissemination is the most frequent metastatic pattern of advanced gastric cancer and the main cause of death in gastric cancer patients. Transforming growth factor-beta1 (TGF- ß1), one of the most potent fibrotic stimuli for human peritoneal mesothelial cells, has been shown to play an important role in this process. In this study, we investigated the effect of TGF- ß1 signaling blockade in gastric cancer cell (GCC)-induced human peritoneal mesothelial cell (HPMC) fibrosis. HPMCs were cocultured with the high TGF- ß1 expressing GCC line SGC-7901 and various TGF- ß1 signaling inhibitors or SGC-7901 transfected with TGF-ß1-specific siRNA. HPMC fibrosis was monitored on the basis of morphology. Expression of the epithelial cell marker, E-cadherin, and the mesenchymal marker, α-smooth muscle actin (α-SMA), was evaluated by Western blotting and immunofluorescence confocal imaging. GCC adhesion to HPMC was also assayed. In nude mouse tumor model, the peritoneal fibrotic status was monitored by immunofluorescent confocal imaging and Masson’s trichrome staining; formation of metastatic nodular and ascites fluid was also evaluated. Our study demonstrated that GCC expressing high levels of TGF-ß1 induced HMPC fibrosis, which is characterized by both upregulation of E-cadherin and downregulation of α-SMA. Furthermore, HPMC monolayers fibrosis was reversed by TGF- ß1 signaling blockade. In vivo, the TGF- ß1 receptor inhibitor SB-431542 partially attenuated early-stage gastric cancer peritoneal dissemination (GCPD). In conclusion, our study confirms the significance of TGFß1 signaling blockade in attenuating GCPD and may provide a therapeutic target for clinical therapy.




Similar content being viewed by others
References
Jayne D. Molecular biology of peritoneal carcinomatosis. Cancer Treat Res. 2007;134:21–33.
Glockzin G, Piso P. Current status and future directions in gastric cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012;21:625–33.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96.
Yonemura Y, Endou Y, Sasaki T, Hirano M, Mizumoto A, Matsuda T, et al. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur J Surg Oncol. 2010;36:1131–8.
Na D, Lv ZD, Liu FN, Xu Y, Jiang CG, Sun Z, et al. Gastric cancer cell supernatant causes apoptosis and fibrosis in the peritoneal tissues and results in an environment favorable to peritoneal metastases, in vitro and in vivo. BMC Gastroenterol. 2012;12:34.
Na D, Lv ZD, Liu FN, Xu Y, Jiang CG, Sun Z, et al. Transforming growth factor beta1 produced in autocrine/paracrine manner affects the morphology and function of mesothelial cells and promotes peritoneal carcinomatosis. Int J Mol Med. 2010;26:325–32.
Lv ZD, Wang HB, Dong Q, Kong B, Li JG, Yang ZC, et al. Mesothelial cells differentiate into fibroblast-like cells under the scirrhous gastric cancer microenvironment and promote peritoneal carcinomatosis in vitro and in vivo. Mol Cell Biochem. 2013;377:177–85.
Lv ZD, Wang HB, Li FN, Wu L, Liu C, Nie G, et al. TGF-ß1 induces peritoneal fibrosis by activating the Smad2 pathway in mesothelial cells and promotes peritoneal carcinomatosis. Int J Mol Med. 2012;29:373–9.
Lv ZD, Na D, Ma XY, Zhao C, Zhao WJ, Xu HM. Human peritoneal mesothelial cell transformation into myofibroblasts in response to TGF-ß1 in vitro. Int J Mol Med. 2011;27:187–93.
Lv ZD, Na D, Liu FN, Du ZM, Sun Z, Li Z, et al. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J Exp Clin Cancer Res. 2010;29:139.
Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-beta superfamily. Cytokine Growth Factor Rev. 1996;7:327–39.
Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25:264–8.
Choi ME, Ding Y, Kim SI. TGF-β signaling via TAK1 pathway: role in kidney fibrosis. Semin Nephrol. 2012;32:244–52.
Pinkas J, Teicher BA. TGF-beta in cancer and as a therapeutic target. Biochem Pharmacol. 2006;72:523–9.
Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–22.
Callahan JF, Burgess JL, Fornwald JA, Gaster LM, Harling JD, Harrington FP, et al. Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5). J Med Chem. 2002;45:999–1001.
Zhou F, Li GY, Gao ZZ, Liu J, Liu T, Li WR, et al. The TGF-ß1/Smad/CTGF pathway and corpus cavernosum fibrous–muscular alterations in rats with streptozotocin-induced diabetes. J Androl. 2012;33:651–9.
Comen EA. Tracking the seed and tending the soil: evolving concepts in metastatic breast cancer. Discov Med. 2012;14:97–104.
Na D, Liu F, Miao Z, Du Z, Xu H. Destruction of gastric cancer cells to mesothelial cells by apoptosis in the early peritoneal metastasis. J Huazhong Univ Sci Technolog Med Sci. 2009;29:163–8.
Na D, Liu FN, Miao ZF, Du ZM, Xu HM. Astragalus extract inhibits destruction of gastric cancer cells to mesothelial cells by anti-apoptosis. World J Gastroenterol. 2009;15:570–7.
Tsukada T, Fushida S, Harada S, Yagi Y, Kinoshita J, Oyama K, et al. The role of human peritoneal mesothelial cells in the fibrosis and progression of gastric cancer. Int J Oncol. 2012;41:476–82.
Togashi Y, Masago K, Fujita S, Kim YH, Sakamori Y, Hatachi Y, et al. Association of the transforming growth factor ß1 promoter polymorphism, C-509T, with smoking status and survival in advanced non-small cell lung cancer. Oncol Rep. 2011;25:377–82.
Ungefroren H, Sebens S, Groth S, Gieseler F, Fändrich F. Differential roles of Src in transforming growth factor-ß regulation of growth arrest, epithelial-to-mesenchymal transition and cell migration in pancreatic ductal adenocarcinoma cells. Int J Oncol. 2011;38:797–805.
Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y, Kato Y, Shinto O, et al. Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFβ signaling. PLoS One. 2013;8:e62310.
Mandel K, Seidl D, Rades D, Lehnert H, Gieseler F, Hass R, et al. Characterization of spontaneous and TGF-β-induced cell motility of primary human normal and neoplastic mammary cells in vitro using novel real-time technology. PLoS One. 2013;8:e56591.
Dhasarathy A, Phadke D, Mav D, Shah RR, Wade PA. The transcription factors Snail and Slug activate the transforming growth factor-beta signaling pathway in breast cancer. PLoS One. 2011;6:e26514.
Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 2009;28:88–98.
Ehata S, Hanyu A, Hayashi M, Aburatani H, Kato Y, Fujime M, et al. Transforming growth factor-beta promotes survival of mammary carcinoma cells through induction of antiapoptotic transcription factor DEC1. Cancer Res. 2007;67:9694–703.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miao, ZF., Zhao, TT., Wang, ZN. et al. Transforming growth factor-beta1 signaling blockade attenuates gastric cancer cell-induced peritoneal mesothelial cell fibrosis and alleviates peritoneal dissemination both in vitro and in vivo. Tumor Biol. 35, 3575–3583 (2014). https://doi.org/10.1007/s13277-013-1472-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1472-x