Abstract
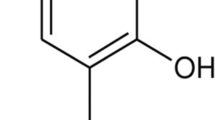
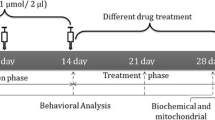
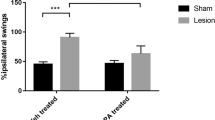
This study was undertaken to investigate the neuroprotective effects of rutin (vitamin P) on 6-hydroxydopamine (6-OHDA)-induced Parkinson’s disease (PD) in rats. Oxidative stress and inflammation is an important event, play a crucial role in neurodegenerative diseases. Rutin has been shown to have antioxidant and anti-inflammatory actions, and thus was tested for its beneficial effects using 6-OHDA-induced PD rat model. Male Wistar rats were pre-treated with rutin (25 mg/kg bwt, orally) for 3 weeks and subjected to unilateral intrastriatal injection of 6-OHDA (10 μg in 0.1% ascorbic acid in normal saline). Three weeks after 6-OHDA infusion, rats were tested for neurobehavioral activity, and were killed after 4 weeks of 6-OHDA infusion for the estimation of thiobarbituric acid reactive substances, glutathione, and its dependent enzymes (glutathione peroxidase and glutathione reductase), dopamine (DA) and its metabolite 3,4-dihydroxyphenyl acetic acid. The increase in 6-OHDA-induced rotations and deficits in locomotor activity and motor coordination and decrease in antioxidant level, DA content and its metabolite and increase in the number of dopaminergic D2 receptors in striatum were protected significantly with lesioned group pre-treated with rutin. These findings were further supported by the histopathological and immunohistochemical findings in the substantia nigra that showed that rutin protected neurons from deleterious effects of 6-OHDA. These results suggest that the consumption of rutin, which is novel vitamin, may have the possibility of protective effect against the neurological disorder such as PD.








Similar content being viewed by others
References
Afanas’av IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovich AI (1989) Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol 38:1763–1769
Agrawal AK, Hussin R, Raghubir R, Kumar A, Seth PK (1995) Neurobehavioural, neurochemical and electrophysiological studies in 6-hydroxydopamine lesioned and neural transplanted rats. Int J Dev Neurosci 13:105–111
Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA et al (2005) Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced Parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J Neurochem 93:94–104
Allbutt HN, Henderson JM (2007) Use of the narrow beam test in the rat, 6-hydroxydopamine model of Parkinson’s disease. J Neurosci Methods 159:195–202
Bishnoi M, Chopra K, Kulkarni SK (2007) Protective effect of rutin, a polyphenolic flavonoid against haloperidol-induced orofacial dyskinesia and associated behavioural, biochemical and neurochemical changes. Fundam Clin Pharmacol 21:521–529
Blandini F, Armentero MT, Martignoni E (2008) The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord 14:S124–S129
Blum D, Torch S, Lambengm N, Nissou M, Benabid A, Sadoul R (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA. Dopamine and MPTP: contribution to apoptotic theory in Parkinson’s disease. Prog Neurobiol 65:135–172
Cannon JR, Keep RF, Hua Y, Richardson RJ, Schallert T, Xi G (2005) Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson’s disease model. Neurosci Lett 373:189–194
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Chaturvedi RK, Shukla S, Seth K, Chauhan S, Sinha C, Shukla Y, Agrawal AK (2006) Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Neurobiol Dis 22:421–434
Choi DK, Pennathur S, Perier C, Tieu K, Teismann P et al (2005) Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci 25:6594–6600
Claiborne A (1985) Catalase activity. In: Green Wald RA (ed) CRC hand book of methods for oxygen radical research. CRC Press, Boca Raton, FL, pp 283–284
del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ (2000) Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol 10:95–112
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Freeman BA, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Investig 47:412–426
Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM (2008) Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28:7687–7698
Gupta R, Singh M, Sharma A (2003) Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol Res 48:209–215
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397
Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP (2003) The role of glial reaction and inflammation in Parkinson’s disease. Ann NY Acad Sci 991:214–228
Hirsch E, Hunot S, Hartmann A (2005) Neuroinflammatory processes in Parkinson’s disease. Parkinsonism Relat Disord 11:S9–S15
Ishrat T, Parveen K, Khan MM, Khuwaja G, Khan MB et al (2009) Selenium prevents cognitive decline and oxidative damage in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res 1281:117–127
Islam F, Zia S, Sayeed I, Zafar KS, Ahmad AS (2002) Selenium induced alteration on lipids, lipid peroxidation, and thiol group in circadian rhythm centers of rat. Biol Trace Elem Res 90:203–214
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53:S26–S36
Jenner P (2007) Oxidative stress and Parkinson’s disease. Handb Clin Neurol 83:507–520
Jiang ZY, Hunt JV, Wolff SP (1992) Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 202:384–389
Jin F, Wu Q, Lu YF, Gong QH, Shi JS (2008) Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol 600:78–82
Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL (2009) The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem 109:656–669
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kamalakkannan N, Prince PSM (2006) Rutin improves the antioxidant status in streptozotocin-induced diabetic rat tissues. Mol Cell Biochem 293:211–219
Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastawa P, Khan MB et al (2009) Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292:123–135
Kirik D, Rosenblad C, Bjorklund A (1998) Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol 152:259–277
Koda T, Kuroda Y, Imai H (2008) Protective effect of rutin against spatial memory impairment induced by trimethyltin in rats. Nutr Res 28:629–634
Koda T, Kuroda Y, Imai H (2009) Rutin supplementation in the diet has protective effects against toxicant-induced hippocampal injury by suppression of microglial activation and pro-inflammatory cytokines: protective effect of rutin against toxicant-induced hippocampal injury. Cell Mol Neurobiol 29:523–531
Koprich JB, Reske-Nielsen C, Mithal P, Isacson O (2008) Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation 5:8
La Casa C, Villegas I, Alarcon De la Lastra C, Motilva V, Martin Calero MJ (2006) Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol 71:45–53
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Li Y, Hu X, Liu Y, Bao Y, An L (2009) Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology 56:580–589
Little JP, Villanueva EB, Klegeris A (2011) Therapeutic potential of cannabinoids in the treatment of neuroinflammation associated with Parkinson’s disease. Mini Rev Med Chem 11:582–590
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263:17205–17208
Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG (1993) A fluorometric assay for the measurement of nitrite in biological samples. Anal Biochem 214:11–16
Mogi M, Togari A, Tanaka K, Ogawa N, Ichinose H, Nagatsu T (1999) Increase in level of tumor necrosis factor (TNF)-alpha in 6-hydroxydopamine-lesioned striatum in rats without influence of systemic L-dopa on the TNF-alpha induction. Neurosci Lett 268:101–104
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller D (1984) Differential distribution of glutathione and glutathione related enzymes in rabbit kidneys: possible implication in analgesic neuropathy. Cancer Res 44:5086–5091
Ogura T, Ogata M, Akita H, Jitsuki S, Akiba L, Noda K et al (2005) Impaired acquisition of skilled behaviour in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci Res 51:299–308
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates, 4th ed. Academic Press, San Diego, CA
Pu F, Mishima K, Egashira N, Iwasaki K, Kaneko T, Uchida T et al (2004) Protective effect of buckwheat polyphenols against long-lasting impairment of spatial memory associated with hippocampal neuronal damage in rats subjected to repeated cerebral ischemia. J Pharmacol Sci 94:393–402
Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K et al (2007) Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci 104:329–334
Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL (1998) The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods 83:165–175
Sánchez-Iglesias S, Méndez-Alvarez E, Iglesias-González J, Muñoz-Patiño A, Sánchez-Sellero I, Labandeira-García JL et al (2009) Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDA-induced model of Parkinson’s disease. J Neurochem 109:879–888
Sauer H, Oertel WH (1994) Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59:401–415
Schwarting RKW, Hudson JL (1996) The unilateral 6-OHDA injection lesion model in behaviour brain research: analysis of functional deficit, recovery and treatment. Prog Neurobiol 50:275–331
Stevens M, Obrosova I, Cao X, Huysen CV, Green DA (2000) Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative stress in experimental diabetic neuropathy. Diabetes 49:1006–1015
Sun Y (1990) Free radicals, antioxidant enzymes and carcinogenesis. Free Rad Biol Med 8:583–599
Tansey MG, McCoy MK, Frank-Cannon TC (2007) Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 208:1–25
Utely HC, Bernheim F, Hochslein P (1967) Effect of sulfhydryl reagent on peroxidation in microsome. Arch Biochem Biophys 260:521–531
Xue YQ, Zhao LR, Guo WP, Duan WM (2007) Intrastriatal administration of erythropoietin protects dopaminergic neurons and improves neurobehavioural outcome in a rat model of Parkinson’s disease. Neuroscience 146:1245–1258
Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F (2003) Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: neurobehavioural and neurochemical evidences. J Neurochem 84:438–446
Acknowledgments
The authors thank the Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH), Ministry of Health and Family Welfare, Government of India, New Delhi, for financial assistance. We greatly acknowledge Ms. Lorie Leo, Department of Internal Medicine, University of Iowa, for reviewing and editing this manuscript. Technical assistance of Dharamvir Singh is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Neurotoxicology Laboratory—Fund for the Improvement of Science and Technology Sponsored by DST and Special Assistance Programme Sponsored by UGC.
Rights and permissions
About this article
Cite this article
Moshahid Khan, M., Raza, S.S., Javed, H. et al. Rutin Protects Dopaminergic Neurons from Oxidative Stress in an Animal Model of Parkinson’s Disease. Neurotox Res 22, 1–15 (2012). https://doi.org/10.1007/s12640-011-9295-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-011-9295-2