Abstract
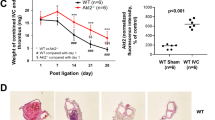
Thrombus age determination in fatal venous thromboembolism cases is an important task for forensic pathologists. In this study, we investigated the time-dependent expressions of formyl peptide receptor 2 (FPR2) and Annexin A1 (ANXA1) in a stasis-induced deep vein thrombosis (DVT) murine model, with the aim of obtaining useful information for thrombus age timing. A total of 75 ICR mice were randomly classified into thrombosis group and control group. In thrombosis group, a DVT model was established by ligating the inferior vena cava (IVC) of mice, and thrombosed IVCs were harvested at 1, 3, 5, 7, 10, 14, and 21 days after modeling. In control group, IVCs without thrombosis were taken as control samples. The expressions of FPR2 and ANXA1 during thrombosis were detected using immunohistochemistry and double immunofluorescence staining. Their protein and mRNA levels in the samples were determined by Western blotting and quantitative real-time PCR. The results reveal that FPR2 was predominantly expressed by intrathrombotic neutrophils and macrophages. ANXA1 expression in the thrombi was mainly distributed in neutrophils, endothelial cells of neovessels, and fibroblastic cells. After thrombosis, the expressions of FPR2 and ANXA1 were time-dependently up-regulated. The percentage of FPR2-positive cells and the level of FPR2 protein significantly elevated at 1, 3, 5 and 7 days after IVC ligation as compared to those at 10, 14 and 21 days after ligation (p < 0.05). Moreover, the mRNA level of FPR2 were significantly higher at 5 days than that at the other post-ligation intervals (p < 0.05). Besides, the levels of ANXA1 mRNA and protein peaked at 10 and 14 days after ligation, respectively. A significant increase in the mRNA level of ANXA1 was found at 10 and 14 days as compared with that at the other post-ligation intervals (p < 0.01). Our findings suggest that FPR2 and ANXA1 are promising as useful markers for age estimation of venous thrombi.






Similar content being viewed by others

Data availability
Data available on request from the authors.
References
Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398:64–77. https://doi.org/10.1016/S0140-6736(20)32658-1.
Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. https://doi.org/10.1007/s11239-015-1311-6.
Ruskin KJ. Deep vein thrombosis and venous thromboembolism in trauma. Curr Opin Anaesthesiol. 2018;31:215–8. https://doi.org/10.1097/ACO.0000000000000567.
Fanola CL, Norby FL, Shah AM, Chang PP, Lutsey PL, Rosamond WD, Cushman M, Folsom AR. Incident heart failure and long-term risk for venous thromboembolism. J Am Coll Cardiol. 2020;75:148–58. https://doi.org/10.1016/j.jacc.2019.10.058.
Byard RW. Deep venous thrombosis, pulmonary embolism and long-distance flights. Forensic Sci Med Pathol. 2019;15:122–4. https://doi.org/10.1007/s12024-018-9991-9.
Cecchi R, Lazzaro A, Catanese M, Mandarelli G, Ferracuti S. Fatal thromboembolism following physical restraint in a patient with schizophrenia. Int J Legal Med. 2012;126:477–82. https://doi.org/10.1007/s00414-012-0670-1.
Irniger W. Histologische altersbestimmung von thrombosen und embolien. Virchows Arch Pathol Anat. 1963;336:220.
Fineschi V, Turillazzi E, Neri M, Pomara C, Riezzo I. Histological age determination of venous thrombosis: a neglected forensic task in fatal pulmonary thrombo-embolism. Forensic Sci Int. 2009;186:22–8. https://doi.org/10.1016/j.forsciint.2009.01.006.
Maffeis V, Nicolè L, Rago C, Fassina A. Histological criteria for age determination of fatal venous thromboembolism. Int J Legal Med. 2018;132:775–80. https://doi.org/10.1007/s00414-017-1705-4.
Bonasoni MP, Muciaccia B, Pelligra CB, Goldoni M, Cecchi R. Third trimester intrauterine fetal death: proposal for the assessment of the chronology of umbilical cord and placental thrombosis. Int J Legal Med. 2022;136:705–11. https://doi.org/10.1007/s00414-022-02784-3.
Nosaka M, Ishida Y, Kimura A, Kondo T. Time-dependent appearance of intrathrombus neutrophils and macrophages in a stasis-induced deep vein thrombosis model and its application to thrombus age determination. Int J Legal Med. 2009;123:235–40. https://doi.org/10.1007/s00414-009-0324-0.
Nosaka M, Ishida Y, Kimura A, Kondo T. Time-dependent organic changes of intravenous thrombi in stasis-induced deep vein thrombosis model and its application to thrombus age determination. Forensic Sci Int. 2010;195:143–7. https://doi.org/10.1016/j.forsciint.2009.12.008.
Nicklas JM, Gordon AE, Henke PK. Resolution of deep venous thrombosis: proposed immune paradigms. Int J Mol Sci. 2020;21:2080. https://doi.org/10.3390/ijms21062080.
Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203:93–8. https://doi.org/10.1016/j.forsciint.2010.07.004.
Nosaka M, Ishida Y, Kimura A, Kondo T. Immunohistochemical detection of MMP-2 and MMP-9 in a stasis-induced deep vein thrombosis model and its application to thrombus age estimation. Int J Legal Med. 2010;124:439–44. https://doi.org/10.1007/s00414-010-0484-y.
Nosaka M, Ishida Y, Kimura A, Hama M, Kawaguchi T, Yamamoto H, Kuninaka Y, Shimada E, Kondo T. Immunohistochemical detection of intrathrombotic IL-6 and its application to thrombus age estimation. Int J Legal Med. 2015;129:1021–5. https://doi.org/10.1007/s00414-015-1147-9.
Prevete N, Liotti F, Marone G, Melillo RM, de Paulis A. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol Res. 2015;102:184–91. https://doi.org/10.1016/j.phrs.2015.09.017.
Lee HY, Lee M, Bae YS. Formyl peptide receptors in cellular differentiation and inflammatory diseases. J Cell Biochem. 2017;118:1300–7. https://doi.org/10.1002/jcb.25877.
Weiß E, Kretschmer D. Formyl-peptide receptors in infection, inflammation, and cancer. Trends Immunol. 2018;39:815–29. https://doi.org/10.1016/j.it.2018.08.005.
Qin CX, Norling LV, Vecchio EA, Brennan EP, May LT, Wootten D, Godson C, Perretti M, Ritchie RH. Formylpeptide receptor 2: nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br J Pharmacol. 2022;179:4617–39. https://doi.org/10.1111/bph.15919.
Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–5. https://doi.org/10.1038/nature12175.
Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Le Y, Gao JL, Zhao J, Wang JM, Wang A. Formylpeptide receptors mediate rapid neutrophil mobilization to accelerate wound healing. PLoS One. 2014;9: e90613. https://doi.org/10.1371/journal.pone.0090613.
Kwon YW, Heo SC, Jang IH, Jeong GO, Yoon JW, Mun JH, Kim JH. Stimulation of cutaneous wound healing by an FPR2-specific peptide agonist WKYMVm. Wound Repair Regen. 2015;23:575–82. https://doi.org/10.1111/wrr.12315.
de Arriba MDC, Fernández G, Chacón-Solano E, Mataix M, Martínez-Santamaría L, Illera N, Carrión-Marchante R, Martín ME, Larcher F, González VM, Del Río M, Carretero M. FPR2 DNA aptamers for targeted therapy of wound repair. J Invest Dermatol. 2022;142:2238-48.e8. https://doi.org/10.1016/j.jid.2021.12.026.
Cattaneo F, Parisi M, Ammendola R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int J Mol Sci. 2013;14:7193–230. https://doi.org/10.3390/ijms14047193.
Huang JJ, Xia CJ, Wei Y, Yao Y, Dong MW, Lin KZ, Yu LS, Gao Y, Fan YY. Annexin A1-derived peptide Ac2-26 facilitates wound healing in diabetic mice. Wound Repair Regen. 2020;28:772–9. https://doi.org/10.1111/wrr.12860.
Senchenkova EY, Ansari J, Becker F, Vital SA, Al-Yafeai Z, Sparkenbaugh EM, Pawlinski R, Stokes KY, Carroll JL, Dragoi AM, Qin CX, Ritchie RH, Sun H, Cuellar-Saenz HH, Rubinstein MR, Han YW, Orr AW, Perretti M, Granger DN, Gavins FNE. Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation. 2019;140:319–35. https://doi.org/10.1161/CIRCULATIONAHA.118.039345.
Vital SA, Senchenkova EY, Ansari J, Gavins FNE. Targeting AnxA1/Formyl Peptide Receptor 2 Pathway Affords Protection against Pathological Thrombo-Inflammation. Cells. 2020;9:2473. https://doi.org/10.3390/cells9112473.
Ansari J, Senchenkova EY, Vital SA, Al-Yafeai Z, Kaur G, Sparkenbaugh EM, Orr AW, Pawlinski R, Hebbel RP, Granger DN, Kubes P, Gavins FNE. Targeting the AnxA1/Fpr2/ALX pathway regulates neutrophil function, promoting thromboinflammation resolution in sickle cell disease. Blood. 2021;137:1538–49. https://doi.org/10.1182/blood.2020009166.
Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE. 2015;10: e0145342. https://doi.org/10.1371/journal.pone.0145342.
Najem MY, Couturaud F, Lemarié CA. Cytokine and chemokine regulation of venous thromboembolism. J Thromb Haemost. 2020;18:1009–19. https://doi.org/10.1111/jth.14759.
Mukhopadhyay S, Johnson TA, Duru N, Buzza MS, Pawar NR, Sarkar R, Antalis TM. Fibrinolysis and Inflammation in Venous Thrombus Resolution. Front Immunol. 2019;10:1348. https://doi.org/10.3389/fimmu.2019.01348.
Humphries J, McGuinness CL, Smith A, Waltham M, Poston R, Burnand KG. Monocyte chemotactic protein-1 (MCP-1) accelerates the organization and resolution of venous thrombi. J Vasc Surg. 1999;30:894–9. https://doi.org/10.1016/s0741-5214(99)70014-5.
Gobbetti T, Cooray SN. Annexin A1 and resolution of inflammation: tissue repairing properties and signalling signature. Biol Chem. 2016;397:981–93. https://doi.org/10.1515/hsz-2016-0200.
Leoni G, Nusrat A. Annexin A1: shifting the balance towards resolution and repair. Biol Chem. 2016;397:971–9. https://doi.org/10.1515/hsz-2016-0180.
McArthur S, Gobbetti T, Kusters DH, Reutelingsperger CP, Flower RJ, Perretti M. Definition of a Novel Pathway Centered on Lysophosphatidic Acid To Recruit Monocytes during the Resolution Phase of Tissue Inflammation. J Immunol. 2015;195:1139–51. https://doi.org/10.4049/jimmunol.1500733.
Maderna P, Cottell DC, Toivonen T, Dufton N, Dalli J, Perretti M, Godson C. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24:4240–9. https://doi.org/10.1096/fj.10-159913.
Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the Resolution of Inflammation: Modulation of Neutrophil Recruitment, Apoptosis, and Clearance. J Immunol Res. 2016;2016:8239258. https://doi.org/10.1155/2016/8239258.
Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, Maderna P. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–605. https://doi.org/10.4049/jimmunol.178.7.4595.
Yi M, Schnitzer JE. Impaired tumor growth, metastasis, angiogenesis and wound healing in annexin A1-null mice. Proc Natl Acad Sci U S A. 2009;106:17886–91. https://doi.org/10.1073/pnas.0901324106.
Lacerda JZ, Drewes CC, Mimura KKO, Zanon CF, Ansari T, Gil CD, Greco KV, Farsky SHP, Oliani SM. Annexin A12–26 Treatment Improves Skin Heterologous Transplantation by Modulating Inflammation and Angiogenesis Processes. Front Pharmacol. 2018;9:1015. https://doi.org/10.3389/fphar.2018.01015.
Bizzarro V, Fontanella B, Carratù A, Belvedere R, Marfella R, Parente L, Petrella A. Annexin A1 N-terminal derived peptide Ac2-26 stimulates fibroblast migration in high glucose conditions. PLoS ONE. 2012;7: e45639. https://doi.org/10.1371/journal.pone.0045639.
Cecchi R. Estimating wound age: looking into the future. Int J Legal Med. 2010;124:523–36. https://doi.org/10.1007/s00414-010-0505-x.
Fan YY, Zhang ST, Yu LS, Ye GH, Lin KZ, Wu SZ, Dong MW, Han JG, Feng XP, Li XB. The time-dependent expression of α7nAChR during skeletal muscle wound healing in rats. Int J Legal Med. 2014;128:779–86. https://doi.org/10.1007/s00414-014-1001-5.
Tian ZL, Jiang SK, Zhang M, Wang M, Li JY, Zhao R, Wang LL, Li SS, Liu M, Zhang MZ, Guan DW. Detection of satellite cells during skeletal muscle wound healing in rats: time-dependent expressions of Pax7 and MyoD in relation to wound age. Int J Legal Med. 2016;130:163–72. https://doi.org/10.1007/s00414-015-1251-x.
Ji XY, Chen Y, Ye GH, Dong MW, Lin KZ, Han JG, Feng XP, Li XB, Yu LS, Fan YY. Detection of RAGE expression and its application to diabetic wound age estimation. Int J Legal Med. 2017;131:691–8. https://doi.org/10.1007/s00414-016-1529-7.
Murase T, Yamamoto T, Koide A, Yagi Y, Kagawa S, Tsuruya S, Abe Y, Umehara T, Ikematsu K. Temporal expression of chitinase-like 3 in wounded murine skin. Int J Legal Med. 2017;131:1623–31. https://doi.org/10.1007/s00414-017-1658-7.
Barington K, Jensen HE, Skovgaard K. Forensic aspects of gene expression signatures for age determination in bruises as evaluated in an experimental porcine model. Forensic Sci Med Pathol. 2017;13:151–60. https://doi.org/10.1007/s12024-017-9869-2.
Abd-Elhakim YM, Omran BHF, Ezzeldein SA, Ahmed AI, El-Sharkawy NI, Mohamed AA. Time-dependent expression of high-mobility group box-1 and toll-like receptors proteins as potential determinants of skin wound age in rats: Forensic implication. Int J Legal Med. 2022;136:1781–9. https://doi.org/10.1007/s00414-022-02788-z.
Kimura A, Ishida Y, NosakaM SM, Hama M, Kawaguchi T, Kuninaka Y, Shimada E, Yamamoto H, Takayasu T, Kondo T. Autophagy in skin wounds: a novel marker for vital reactions. Int J Legal Med. 2015;129:537–41. https://doi.org/10.1007/s00414-015-1168-4.
Pennisi G, Torrisi M, Cocimano G, Esposito M, Salerno M, Sessa F. Vitality markers in forensic investigations: a literature review. Forensic Sci Med Pathol. 2023;19:103–16. https://doi.org/10.1007/s12024-022-00551-9.
Xie DG, Wang XM, Li JH, Tan ZY, Zhang ZQ, Li ST. Short-term postmortem interval estimation by detection of apoptosis-related protein in skin. Forensic Sci Med Pathol. 2024. https://doi.org/10.1007/s12024-023-00757-5.
Nosaka M, Ishida Y, Kuninaka Y, Kimura A, Kondo T. Immunohistochemical detection of uPA, tPA, and PAI-1 in a stasis-induced deep vein thrombosis model and its application to thrombus age estimation. Int J Legal Med. 2012;126:421–5. https://doi.org/10.1007/s00414-012-0680-z.
Nosaka M, Ishida Y, Kuninaka Y, Taruya A, Kimura A, Shimada E, Yamamoto H, Michiue T, Furukawa F, Kondo T. The application of autophagy to thrombus age estimation in murine deep vein thrombosis model. Int J Legal Med. 2020;134:1061–6. https://doi.org/10.1007/s00414-019-02168-0.
Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–73. https://doi.org/10.1016/S0140-6736(16)30514-1.
Engbers MJ, van Hylckama VA, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemos. 2010;8:2105–12. https://doi.org/10.1111/j.1538-7836.2010.03986.x.
Wang H, Rosendaal FR, Cushman M, van Hylckama VA. Procoagulant factor levels and risk of venous thrombosis in the elderly. J Thromb Haemost. 2021;19:186–93. https://doi.org/10.1111/jth.15127.
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province in China (LY20H150006) and National Natural Science Foundation of China (81301640).
Author information
Authors and Affiliations
Contributions
Yan-Yan Fan and Peng-Fei Jiang designed the research and wrote the manuscript. Jun-Jie Huang and Jia-ying Zhuo conducted the animal experiments, sample collection, Western blot and Real time PCR. Measurement of thrombus size was conducted by Qian Wang, Yue Sun, and Jia-Xin Qi. Immunohistochemistry, morphometrical analysis, and double immunofluorescence staining were performed by Juan-Juan Wu, Qian Wang, Yue Sun, Jia-Xin Qi, and Yu Zhang. Experimental data were analyzed by Gang Chen. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All animal work in this study was approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University.
Informed consent
No informed consent was required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, JJ., Zhuo, Jy., Wang, Q. et al. The time-dependent expression of FPR2 and ANXA1 in murine deep vein thrombosis model and its relation to thrombus age. Forensic Sci Med Pathol (2024). https://doi.org/10.1007/s12024-024-00818-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12024-024-00818-3