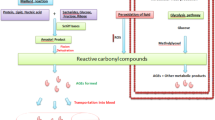
Abstract
The vascular diseases, hypertension and atherosclerosis, affect millions of individuals worldwide, and account for a large number of deaths globally. A better understanding of the mechanism of these conditions will lead to more specific and effective therapies. Hypertension and atherosclerosis are both characterized by insulin resistance, and we suggest that this plays a major role in their etiology. The cause of insulin resistance is not known, but may be a result of a combination of genetic and lifestyle factors. In insulin resistance, alterations in glucose and lipid metabolism lead to the production of excess aldehydes including glyoxal and methylglyoxal. These aldehydes react non-enzymatically with free amino and sulfhydryl groups of amino acids of proteins to form stable conjugates called advanced glycation end products (AGEs). AGEs act directly, as well as via receptors to alter the function of many intra- and extracellular proteins including antioxidant and metabolic enzymes, calcium channels, lipoproteins, and transcriptional and structural proteins. This results in endothelial dysfunction, inflammation and oxidative stress. All these changes are characteristic of hypertension and atherosclerosis. Human and animal studies have demonstrated that increased AGEs are also associated with these conditions. A pathological role for AGEs is substantiated by studies showing that therapies that attenuate insulin resistance and/or lower AGEs, are effective in decreasing oxidative stress, lowering blood pressure, and attenuating atherosclerotic vascular changes. These interventions include lipoic acid and other antioxidants, AGE breakers or soluble receptors of AGEs, and aldehyde-binding agents like cysteine. Such therapies may offer alternative specific means to treat hypertension and atherosclerosis. An adjunct therapy may be to implement lifestyle changes such as weight reduction, regular exercise, smoking cessation, and increasing dietary intake of fruits and vegetables that also decrease insulin resistance as well as oxidative stress.




Similar content being viewed by others
References
World Health Organization. (2007). Global strategy on diet, physical activity and health: Chronic disease risk factors. http://www.who.int/dietphysicalactivity/publications/facts/riskfactors/en/print.html (accessed February 19, 2007).
World Hypertension League. (2007). Know your blood pressure. http://hsc.utoledo.edu/org/whl/know.html (accessed February 27, 2007).
Taddei, S., Virdis, A., Ghiadoni, L., Salvetti, G., & Salvetti, A. (2000). Endothelial dysfunction and hypertension. Journal of Nephrology, 13, 205–210.
Portaluppi, F., Boari, B., & Manfredini, R. (2004). Oxidative stress in essential hypertension. Current Pharmaceutical Design, 10, 1695–1698.
Resnick, L. M. (1993). Ionic basis of hypertension, insulin resistance, vascular disease, and related disorders. The mechanism of “syndrome X”. American Journal of Hypertension, 6, 123S–134S.
World Health Organization. (2007). Global strategy on diet, physical activity and health: Cardiovascular disease: Prevention and control. http://www.who.int/dietphysicalactivity/publications/facts/cvd/en/print.html (accessed February 19, 2007).
Ross, R. (1999). Atherosclerosis – an inflammatory disease. The New England Journal of Medicine, 340, 115–126.
Tegos, T. J., Kalodiki, E., Sabetai, M. M., & Nicolaides, A. N. (2001). The genesis of atherosclerosis and risk factors: A review. Angiology, 52, 89–98.
Aronow, W. S. (2005). Management of peripheral arterial disease. Cardiology in Review, 13, 61–68.
Pepine, C. J., & Handberg, E. M. (2001). The vascular biology of hypertension and atherosclerosis and intervention with calcium antagonists and angiotensin-converting enzyme inhibitors. Clinical Cardiology, 24, V1–V5.
Reaven, G. M. (2003). Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. The Journal of Clinical Endocrinology and Metabolism, 88, 2399–2403.
Ferrannini, E., Buzzigoli, G., Bonadonna, R., Giorico, M. A., Oleggini, M., Graziadei, L., Pedrinelli, R., Brandi, L., & Bevilacqua, S. (1987). Insulin resistance in essential hypertension. The New England Journal of Medicine, 317, 350–357.
Howard, G., O’Leary, D. H., Zaccaro, D., Haffner, S., Rewers, M., Hamman, R., Selby, J. V., Saad, M. F., Savage, P., & Bergman, R. (1996). Insulin sensitivity and atherosclerosis. Circulaion, 93, 1809–1817.
Suzuki, M., Ikebuchi, M., Shinozaki, K., Hara, Y., Tsushima, M., Matsuyama, T., & Harano, Y. (1996). Mechanism and clinical implication of insulin resistance syndrome. Diabetes, 45, S52–S54.
Harano, Y., Suzuki, M., Shinozaki, K., Hara, Y., Ryomoto, K., Kanazawa, A., Nishioheda, Y., & Tsushima, M. (1996). Clinical impact of insulin resistance syndrome in cardiovascular diseases and its therapeutic approach. Hypertension Research, 19, S81–S85.
Alexander, M. C., Lomanto, M., Nasrin, N., & Ramaika, C. (1988). Insulin stimulates glyceraldehyde-3-phosphate dehydrogenase gene expression through cis-acting DNA sequences. Proceedings of the National Academy of Sciences, 85, 5092–5096.
Thornalley, P. J. (1993). Modification of the glyoxalase system in disease processes and prospects for therapeutic strategies. Biochemical Society Transactions, 21, 531–534.
Beisswenger, P. J., Howell, S. K., Smith, K., & Szwergold, B. S. (2003). Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochimica et Biophysica Acta, 1637, 98–106.
Avramoglu, R. K., Basciano, H., & Adeli, K. (2006). Lipid and lipoprotein dysregulation in insulin resistant states. Clinica Chimica Acta, 368, 1–19.
Howard, B. V. (1999). Insulin resistance and lipid metabolism. American Journal of Cardiology, 84, 28J–32J.
Stocker, R., & Keaney, J. F. Jr. (2004). Role of oxidative modifications in atherosclerosis. Physiological Reviews, 84, 1381–1478.
Touyz, R. M., & Schiffrin, E. L. (2004). Reactive oxygen species in vascular biology: Implications for hypertension. Histochemistry and Cell Biology, 122, 339–352.
Catala, A. (2006). An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. International Journal of Biochemistry & Cell Biology, 38, 1482–1495.
Niki, E., Yoshida, Y., Saito, Y., & Noguchi, N. (2005). Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochemical and Biophysical Research Communications, 338, 668–676.
Uchida, K. (2000). Role of reactive aldehyde in cardiovascular disease. Free Radical Biology and Medicine, 28, 1685–1696.
Schauenstein, E., Esterbauer, H., & Zollner, H. (1977). Aldehydes in biological systems. In J. R. Lagnado (Ed.), Aldehydes in biological systems, their natural occurrence and biological activities (pp. 1–7). London, UK: Pion Limited.
Thornalley, P. J. (2003). Glyoxalase I – structure, function and a critical role in the enzymatic defence against glycation. Biochemical Society Transactions, 31, 1343–1348.
Zeng, J., & Davies, M. J. (2005). Evidence for the formation of adducts and S-(carboxymethyl)cysteine on reaction of α-dicarbonyl compounds with thiol groups on amino acids, peptides, and proteins. Chemical Research in Toxicology, 18, 1232–1241.
Thornalley, P. J., Battah, S., Ahmed, N., Karachalias, N., Agalou, S., Babaei-Jadidi, R., & Dawnay, A. (2003). Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spec. Biochemical Journal, 375, 581–592.
Thorpe, S. R., & Baynes, J. W. (2003). Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 25, 275–281.
Baynes, J. W., & Thorpe, S. R. (2000). Glycoxidation and lipoxidation in atherogenesis. Free Radical Biology and Medicine, 28, 1708–1716.
Morgan, P. E., Dean, R. T., & Davies, M. J. (2002). Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Archives of Biochemistry and Biophysics, 403, 259–269.
Park, Y. S., Koh, Y. H., Takahashi, M., Miyamoto, Y., Suzuki, K., Dohmae, N., Takio, K., Honke, K., & Taniguchi, N. (2003). Identification of the binding site of methylglyoxal on glutathione peroxidase: Methylglyoxal inhibits glutathione peroxidase activity via binding to glutathione binding sites Arg 184 and 185. Free Radical Research, 37, 205–211.
Bidasee, K. R., Zhang, Y., Shao, C. H., Wang, M., Patel, K. P., Sincer, U. D., & Besch, H. R. Jr. (2004). Diabetes increases formation of advanced glycation end products on sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes 53, 463–473.
Riboulet-Chavey, A., Peirron, A., Durand, I., Murdaca, J., Giudicelli, J., van Obberghen, E. (2006). Methylglyoxal impairs the insulin signaling pathways independently of the formation of intracellular reactive oxygen species. Diabetes 55, 1289–1299.
Jia, X., Olson, D. J. H., Ross, A. R. S., & Wu, L. (2006). Structural and functional changes in human insulin induced by methylglyoxal. FASEB Journal, 20, E871–E879.
Tanji, N., Markowitz, G. S., Fu, C., Kislinger, T., Taguchi, A., Pischetsrieder, M., Stern, D., Schmidt, A. M., & D’Agati, V. D. (2000). Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. Journal of the American Society of Nephrology, 11, 1656–1666.
Misselwitz, J., Franke, S., Kauf, E., John, U., & Stein, G. (2002). Advanced glycation end products in children with chronic renal failure and type 1 diabetes. Pediatric Nephrology, 17, 316–321.
Hirata, K., & Kubo, K. (2004). Relationship between blood levels of N-carboxymethyl-lysine and pentosidine and the severity of microangiopathy in Type 2 diabetes. Endocrine Journal, 51, 537–544.
Oya, T., Hattori, N., Mizuno, Y., Miyata, S., Maeda, S., Osawa, T., & Uchida, K. (1999). Methylglyoxal modification of protein. Journal of Biological Chemistry, 274, 18492–18502.
Lieuw-A-Fa, M. L. M., van Hinsbergh, V. W. M., Teerlink, T., Barto, R., Twisk, J., Stehouwer, C. D. A., & Schalkwijk, C. G. (2004). Increased levels of Nɛ-(carboxymethyl)lysine and Nɛ-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: Correlation with markers of endothelial function. Nephrology Dialysis Transplantation, 19, 631–636.
Thornalley, P. J. (2005). Dicarbonyl intermediates in the maillard reaction. Annals of the New York Academy of Sciences, 1043, 111–117.
Alt, N., Carson, J. A., Alderson, N. L., Wang, Y., Nagai, R., Henle, T., Thorpe, S. R., & Baynes, J. W. (2004). Chemical modification of muscle protein in diabetes. Archives of Biochemistry and Biophysics, 425, 200–206.
Mostafa, A. A., Randell, E. W., Vasdev, S. C., Gill, V. D., Han, Y., Gadag, V., Raouf, A. A., & El Said, H. (2007). Plasma protein advanced glycation end products, carboxymethyl cysteine and carboxyethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Molecular and Cellular Biochemistry, DOI: 10.1007/s11010-007-9422-9.
Chen, P. F., Tsai, A. L., & Wu, K. K. (1994). Cysteine 184 of endothelial nitric oxide synthase is involved in heme coordination and catalytic activity. Journal of Biological Chemistry, 269, 25062–25066.
Stadtman, T. C. (1990). Selenium biochemistry. Annual Review of Biochemistry, 59, 111–127.
Suzuki, Y. J., & Ford, G. D. (1991). Inhibition of Ca2+-ATPase of vascular smooth muscle sarcoplasmic reticulum by reactive oxygen intermediates. American Journal of Physiology, 261, H568–H574.
Nakashima, I., Takeda, K., Kawamoto, Y., Okuno, Y., Kato, M., & Suzuki, H. (2005). Redox control of catalytic activities of membrane-associated protein tyrosine kinases. Archives of Biochemistry and Biophysics, 434, 3–10.
Avirim, M., Billecke, S., Sorenson, R., Bisgaier, C., Newton, R., Rosenblat, M., Erogul, J., His, C., Dunlop, C., & La Du, B. (1998). Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activites. Selective action of human paraoxonase allozymes Q, & R. Arteriosclerosis Thrombosis and Vascular Biology, 18, 1617–1624.
Zaidi, N. F., Lagenaur, C. F., Abramson, J. J., Pessah, I., & Salama, G. (1989). Reactive disulfides trigger Ca2+ release from sarcoplasmic reticulum via an oxidation reaction. Journal of Biological Chemistry, 264, 21725–21736.
Liu, H., Colavitti, R., Rovira, I. I., & Finkel, T. (2005). Redox-dependent transcriptional regulation. Circulation Research, 97, 967–974.
Schmidt, A. M., Yan, S. D., Yan, S. F., & Stern, D. M. (2001). The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. Journal of Clinical Investigation, 108, 949–955.
Bierhaus, A., Humpert, P. M., Morcos, M., Wendt, T., Chavakis, T., Arnold, B., Stern, D. M., & Nawroth, P. P. (2005). Understanding RAGE, the receptor for advanced glycation end products. Journal of Molecular Medicine, 83, 876–886.
Rashid, G., Benchetrit, S., Fishman, D., & Bernheim, J. (2004). Effect of advanced glycation end-products on gene expression and synthesis of TNF-α and endothelial nitric oxide synthase by endothelial cells. Kidney International, 66, 1099–1106.
Scivittaro, V., Ganz, M. B., & Weiss, M. F. (2000). AGEs induce oxidative stress and activate protein kinase C-BII in neonatal mesangial cells. American Journal of Physiology (Renal Physiology), 278, F676–F683.
Reddy, M. A., Li, S. L., Sahar, S., Kim, Y. S., Xu, Z. G., Lanting, L., & Natarajan, R. (2006). Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. Journal of Biological Chemistry, 281, 13685–13693.
Collison, K. S., Parhar, R. S., Saleh, S. S., Meyer, B. F., Kwaasi, A. A., Hammami, M. M., Schmidt, A. M., Stern, D. M., & Al-Mohanna, F. (2002). RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). Journal of Leukocyte Biology, 71, 433–444.
Gu, L., Hagiwara, S., Fan, Q., Tanimoto, M., Kobata, M., Yamashita, M., Nishitani, T., Gohda, T., Ni, Z., Qian, J., Horikoshi, S., & Timono, Y. (2006). Role of receptor for advanced glycation end-products and signaling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrology Dialysis Transplantation, 21, 299–313.
Horiuchi, S., Sakamoto, Y., & Sakai, M. (2003). Scavenger receptors for oxidized and glycated proteins. Amino Acids, 25, 283–292.
Yan, S. D., Schmidt, A. M., Anderson, G. M., Zhang, J., Brett, J., Zou, Y. S., Pinsky, D., & Stern, D. (1994). Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. Journal of Biological Chemistry, 269, 9889–9897.
Cohen, M. P., Shea, E., Chen, S., & Shearman, C. W. (2003). Glycated albumin increases oxidative stress, activates NF-κB and extracellular signal-regulated kinase (ERK), and stimulates ERK-dependent transforming growth factor-β1 production in macrophage RAW cells. Journal of Laboratory and Clinical Medicine, 141, 242–249.
Bierhaus, A., Chevion, S., Chevion, M., Hofmann, M., Quehenberger, P., Illmer, T., Luther, T., Berentshtein, E., Tritschler, H., Muller, M., Wahl, P., Ziegler, R., & Nawroth, P. P. (1997). Advanced glycation end product-induced activation of NF-kB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes, 46, 1481–1490.
Kunt, T., Forst, T., Wilhelm, A., Tritschler, H., Pfuetzner, A., Harzer, O., Engelbach, M., Zschaebitz, A., Stofft, E., & Beyer, J. (1999). α-Lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clinical Science, 96, 75–82.
Wautier, M. P., Chappey, O., Corda, S., Stern, D. M., Schmidt, A. M., & Wautier, J. L. (2001). Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. American Journal of Physiology (Endocrinology and Metabolism), 280, E685–E694.
Haberland, M. E., Fless, G. M., Scanu, A. M., & Fogelman, A. M. (1992). Malondialdehyde modification of lipoprotein(a) produces avid uptake by human monocyte-macrophages. Journal of Biological Chemistry, 267, 4143–4151.
Kirstein, M., Aston, C., Hintz, R., & Vlassara, H. (1992). Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product – modified proteins. Journal of Clinical Investigation, 90, 439–446.
Vasdev, S., Ford, C. A., Parai, S., Longerich, L., & Gadag, V. (2000). Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutrition, Metabolism, and Cardiovascular Diseases, 10, 339–346.
Vasdev, S., Ford, C. A., Parai, S., Longerich, L., & Gadag, V. (2000). Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. Journal of Hypertension, 18, 567–573.
Stitt, A. W., Frizzel, N., & Thorpe, S. R. (2004). Advanced glycation and advanced lipoxidation: Possible role in initiation and progression of diabetic retinopathy. Current Pharmaceutical Design, 10, 3349–3360.
Takeuchi, M., & Yamagishi, S. (2004). TAGE (toxic AGEs) hypothesis in various chronic diseases. Medical Hypothesis, 63, 449–452.
Zieman, S. J., & Kass, D. A. (2004). Advanced glycation endproduct crosslinking in the cardiovascular system. Drugs, 64, 459–470.
Wautier, J. L., & Schmidt, A. M. (2004). Protein glycation. A firm link to endothelial cell dysfunction. Circulation Research, 95, 233–238.
Vasdev, S., Gill, V., & Longerich, L. (2004). Role of methylglyoxal in essential hypertension. In S. K. Gupta, et al. (Eds.), Pharmacotherapy of heart failure (pp. 72–88). New Delhi, India: Anamaya Publishers.
Wu, L. (2006). Is methylglyoxal a causative factor for hypertension development? Canadian Journal of Physiology and Pharmacology, 84, 129–139.
Beisswenger, P. J., Moore, L. L., Brinck-Johnsen, T., & Curphey, T. J. (1993). Increased collagen-linked pentosidine levels and advanced glycosylation end products in early diabetic nephropathy. Journal of Clinical Investigation, 92, 212–217.
Aso, Y., Inukai, T., Tayama, K., & Takemura, Y. (2000). Serum concentrations of advanced glycation end products are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetologia, 37, 87–92.
Alderson, N. L., Chachich, M. E., Youssef, N. N., Beattie, J., Nachtigal, M., Thorpe, S. R., & Baynes, J. W. (2003). The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease. Kidney International, 63, 2123–2133.
Degenhardt, T. P., Alderson, N. L., Arrington, D., Beattie, R. J., Basgen, J. M., Steffes, M. W., Thorpe, S. R., & Baynes, J. W. (2002). Pyridoxamine inhibits early renal disease and dyslipidemia in streptozotocin-diabetic rat. Kidney International, 61, 939–950.
Fosmark, D. S., Torjesen, P. A., Kilhovd, B. K., Berg, T. J., Sandvik, L., Hanssen, K. F., Agardh, C. D., & Agardh, E. (2006). Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism: Clinical and Experimental, 55, 232–236.
Metz, T. O., Alderson, N. L., Thorpe, S. R., & Baynes, J. W. (2003). Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: A novel therapy for treatment of diabetic complications. Archives of Biochemistry and Biophysics, 419, 41–49.
Miyata, T., Ueda, Y., Shinzato, T., Iida, Y., Tanaka, S., Kurokawa, K., van Ypersels de Strihou, C., & Maeda, K. (1996). Accumulation of albumin-linked and free-form pentosidine in the circulation of uremic patients with end-stage renal failure: Renal implications in the pathophysiology of pentosidine. Journal of the American Society of Nephrology, 7, 1198–1206.
Nagaraja, R. H., Sarkar, P., Mally, A., Biemel, K. M., Lederer, M. O., & Padayatti, P. S. (2002). Effect of pyridoxamine on chemical modification of protein by carbonyls in diabetic rats: Characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Archives of Biochemistry and Biophysics, 402, 110–119.
Karachalias, N., Babaei-Jadidi, R., Ahmed, N., & Thornalley, P. J. (2003). Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rat. Biochemical Society Transactions, 31, 1423–1425.
Babaei-Jadidi, R., Karachalias, N., Ahmed, N., Battah, S., & Thornalley, P. J. (2003). Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes, 52, 2110–2120.
Park, L., Raman, K. G., Lee, K. J., Lu, Y., Ferran, L. J. Jr., Chow, W. S., Stern, D., & Schmidt, A. M. (1998). Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nature Medicine, 4, 1025–1031.
Bucciarelli, L. G., Wendt, T., Qu, W., Lu, Y., Lalla, E., Rong, L. L., Goova, M. T., Moser, B., Kislinger, T., Lee, D. C., Kashyap, Y., Stern, D. M., & Schmidt, A. M. (2002). RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation, 106, 2827–2835.
Myint, K. M., Yamamoto, Y., Sakurai, S., Harashima, A., Watanabe, T., Li, H., Takeuchi, A., Yoshimura, K., Yonekura, H., & Yamamoto, H. (2005). Blockade of diabetic vascular injury by controlling of AGE-RAGE system. Current Drug Targets, 6, 447–452.
Stitt, A. W., He, C., Friedman, S., Scher, L., Rossi, P., Ong, L., Founds, H., Li, Y. M., Bucala, R., & Vlassara, H. (1997). Elevated AGE-modified ApoB in sera of euglycemic, normolipidemic patients with atherosclerosis: Relationship to tissue AGEs. Molecular Medicine, 3, 617–627.
Palinski, W., Koschinsky, T., Butler, S. W., Miller, E., Vlassara, H., Cerami, A., & Witztum, J. L. (1995). Immunological evidence for the presence of advanced glycosylation end products in atherosclerotic lesions of euglycemic rabbits. Arteriosclerosis Thrombosis and Vascular Biology, 15, 571–582.
Sima, A., & Stancu, C. (2002). Modified lipoproteins accumulate in human coronary atheroma. Journal of Cellular and Molecular Medicine, 6, 110–111.
Nagai, R., Hayashi, C. M., Xia, L., Takeya, M., & Horiuchi, S. (2002). Identification in human atherosclerotic lesions of GA-pyridine, a novel structure derived from glycoaldehyde-modified proteins. Journal of Biological Chemistry, 277, 48905–48912.
Imanaga, Y., Sakata, N., Takebayashi, S., Matsunaga, A., Sasaki, J., Arakawa, K., Nagai, R., Horiuchi, S., Itabe, H., & Takano, T. (2000). In vivo and in vitro evidence for the glycoxidation of low density lipoprotein in human atherosclerotic plaques. Atherosclerosis, 15, 343–355.
Midaoui, A. E. L., Elimadi, A., Wu, L., Haddad, P. S., de Champlain, J. (2003). Lipoic acid prevents hypertension, hyperglycemia and the increase in heart mitochondrial superoxide production. American Journal of Hypertension, 16, 173–179.
Wang, X., Desai, K., Chang, T., & Wu, L. (2005). Vascular methylglyoxal metabolism and the development of hypertension. Journal of Hypertension, 23, 1565–1573.
Vasdev, S., Ford, C. A., Longerich, L., Parai, S., Gadag, V., & Wadhawan, S. (1998). Aldehyde induced hypertension in rats: Prevention by N-acetylcysteine. Artery, 23, 10–36.
Sugiyama, S., Miyata, T., Ueda, Y., Tanaka, H., Maeda, K., Kawashima, S., Van Ypersele de Strihou, C., & Kurokawa, K. (1998). Plasma levels of pentosidine in diabetic patients: An advanced glycation end product. Journal of the American Society of Nephrology, 9, 1681–1688.
Cooke, C. L. M., Brockelsby, J. C., Baker, P. N., & Davidge, S. T. (2003). The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertension in Pregnancy, 22, 173–184.
Geroldi, D., Falcone, C., Emanuele, E., D’Angelo, A., Calcagnino, M., Buzzi, M. P., Scioli, G. A., & Fogari, R. (2005). Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. Journal of Hypertension, 23, 1725–1729.
Zieman, S. J., & Kass, D. A. (2004). Advanced glycation endproduct crosslinking in the cardiovascular system. Potential therapeutic target for cardiovascular disease. Drugs, 64, 459–470.
Aronson, D. (2003). Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. Journal of Hypertension, 21, 3–12.
McNulty, M., Mahmud, A., & Feely, J. (2007). Advanced glycation end-products and arterial stiffness in hypertension. American Journal of Hypertension, 20, 242–247.
Schram, M. T., Schalkwijk, C. G., Bootsma, A. H., Fuller, J. H., Chaturvedi, N., Stehouwer, C. D. A. (2005). Advanced glycation end products are associated with pulse pressure in Type 1 diabetes. The EURODIAB Prospective Complications study. Hypertension, 46, 232–237.
Safar, M. E. (2001). Systolic blood pressure, pulse pressure and arterial stiffness as cardiovascular risk factors. Current Opinion in Nephrology and Hypertension, 10, 257–261.
Wolffenbuttel, B. H. R., Boulanger, C. M., Crijns, F. R. L., Huijberts, M. S. P., Poitevin, P., Swennen, G. N. M., Vasan, S., Egan, J. J., Ulrich, P., Cerami, A., Levy, B. I. (1998). Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proceedings of the National Academy of Sciences, 95, 4630–4634.
Kass, D. A., Shapiro, E. P., Kawaguchi, M., Capriotti, A. R., Scuteri, A., deGroof, R. C., Lakatta, E. G. (2001). Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation, 104, 1464–1470.
Zieman, S. J., Melenovsky, V., Clattenberg, L., Corretti, M. C., Capriotti, A., Gerstenblith, G., & Kass, D. A. (2007). Advanced glycation endproduct crosslink breaker (alagebrium) improves endothelial function in patients with isolated systolic hypertension. Journal of Hypertension, 25, 577–583.
Bakris, G. L., Bank, A. J., Kass, D. A., Neutel, J. M., Preston, R. A., & Oparil, S. (2004). Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. American Journal of Hypertension, 17, 23S–30S.
Vasdev, S., & Gill, V. (2005). Antioxidants in the treatment of hypertension. International Journal of Angiology, 14, 60–73.
Mizutani, K., Ikeda, K., Tsuda, K., & Yamori, Y. (2002). Inhibitor for advanced glycation end product formation attenuates hypertension and oxidative stress in genetic hypertensive rats. Journal of Hypertension, 20, 1607–1614.
Anderson, M. M., Requena, J. R., Crowley, J. R., Thorpe, S. R., & Heinecke, J. W. (1999). The myeloperoxidase system in human phagocytes generates Nɛ-(carboxymethyl)lysine on proteins: A mechanism for producing advanced glycation end products at sites of inflammation. Journal of Clinical Investigation, 104, 103–113.
Wu, L. (2005). The pro-oxidant role of methylglyoxal in mesenteric artery smooth muscle cells. Canadian Journal of Physiology and Pharmacology, 83, 63–68.
Zhang, M., Kho, A. L., Anilkumar, N., Chibber, R., Pagano, P. J., Shah, A. M., & Cave, A. C. (2006). Glycated proteins stimulate reactive oxygen species production in cardiac myocytes. Involvement of NOX2 (gp91phox)-containing NADPH oxidase. Circulation, 113, 1235–1243.
Verbeke, P., Perichon, M., Friguet, B., & Bakala, H. (2000). Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochimica et Biophysica Acta, 1502, 481–494.
Xu, B., Ji, Y., Yao, K., Cao, Y. X., & Ferro, A. (2005). Inhibition of human endothelial cell nitric oxide synthesis by advanced glycation end-products but not glucose: Relevance to diabetes. Clinical Science, 109, 439–446.
Rojas, A., Romay, S., Gonzalez, D., Herrera, B., Delgado, R., & Otero, K. (2000). Regulation of endothelial nitric oxide synthase expression by albumin-derived advanced glycosylation end products. Circulation Research, 86, e50–e54.
Chakravarthy, U., Hayes, R. G., Stitt, A. W., McAuley, E., & Archer, D. B. (1998). Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes, 47, 945–952.
Jan, C. R., Chen, C. H., Wang, S. C., & Kuo, S. Y. (2005). Effect of methylglyoxal on intracellular calcium levels and viability in renal tubular cells. Cell Signalling, 17, 847–855.
Yamagishi, S. I., Fujimori, H., Yonekura, H., Tanaka, N., & Yamamoto, H. (1999). Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochemical and Biophysical Research Communications, 258, 353–357.
Kislinger, T., Tanji, N., Wendt, T., Qu, W., Lu, Y., Ferran, L. J., Taguchi, A., Olson, K., Bucciarelli, L., Goova, M., Hofmann, M. A., Cataldegirmen, G., D’Agati, V., Pischetsrieder, M., Stern, D. M., & Schmidt, A. M. (2001). Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arteriosclerosis Thrombosis and Vascular Biology, 21, 905–910.
Jenkins, A. J., Velarde, V., Klein, R. L., Joyce, K. C., Phillips, K. D., Mayfield, R. K., Lyons, T. J., & Jaffa, A. A. (2000). Native and modified LDL activate extracellular signal-regulated kinases in mesangial cells. Diabetes, 49, 2160–2169.
Velarde, V., Jenkins, A. J., Christopher, J., Lyons, T. J., & Jaffa, A. A. (2001). Activation of MAPK by modified low-density lipoproteins in vascular smooth muscle cells. Journal of Applied Physiology, 91, 1412–1420.
De Nigris, D., Gallo, L., Sica, V., & Napoli, C. (2006). Glycoxidation of low-density lipoprotein promotes multiple apoptotic pathways and NFkB activation in human coronary cells. Basic Research in Cardiology, 101, 101–108.
Lee, H. J., Howell, S. K., Sanford, R. J., & Beisswenger, P. J. (2005). Methylglyoxal can modify GAPDH activity and structure. Annals of the New York Academy of Sciences, 1043, 135–145.
Chang, K. C., Paek, K. S., Kim, H. J., Lee, Y. S., Yabe-Nishimura, C., & Seo, H. G. (2002). Substrate-induced up-regulation of aldose reductase by methylglyoxal, a reactive oxoaldehyde elevated in diabetes. Molecular Pharmacology, 61, 1184–1191.
Miele, C., Riboulet, A., Maitan, M. A., Oriente, F., Romano, C., Formisano, P., Giudicelli, J., Beguinot, F., & Van Obberghen, E. (2003). Human glycated albumin affects glucose metabolism in L6 skeletal muscle cells by impairing insulin-induced insulin receptor substrate (IRS) signaling through a protein kinase C α-mediated mechanism. Journal of Biological Chemistry, 278, 47376–47387.
Chang, S. G., Choi, K. D., Jang, S. H., & Shin, H. C. (2003). Role of disulfide bonds in the structure and activity of human insulin. Molecular Cell, 16, 323–330.
McKillop, A. M., Abdel-Wahab, Y. H. A., Mooney, M. H., O’Harte, F. P. M., & Flatt, P. R. (2002). Secretion of glycated insulin from pancreatic β-cells in diabetes represent a novel aspect of β-cell dysfunction and glucose toxicity. Diabetes & Metabolism, 28, 3S62–3S69.
Lindsay, J. R., McKillop, A. M., Mooney, M. H., O’Harte, F. P. M., Bell, P. M., & Flatt, P. R. (2003). Demonstration of increased concentrations of circulating glycated insulin in human Type 2 diabetes using a novel and specific radioimmunoassay. Diabetologia, 46, 475–478.
Abdel-Wahab, Y. H. A., O’Harte, F. P. M., Boyd, A. C., Barnett, C. R., & Flatt, P. R. (1997). Glycation of insulin results in reduced biological activity in mice. Acta Diabetologia, 34, 265–270.
McKillop, A. M., Mooney, M. H., Harriott, P., Flatt, P. R., & O’Harte, F. P. M. (2001). Evaluation of glycated insulin in diabetic animals using immunocytochemistry and radioimmunoassay. Biochemical and Biophysical Research Communications, 286, 524–528.
Hunter, S. J., Boyd, A. C., O’Harte, F. P. M., McKillop, A. M., Wiggam, M. I., Mooney, M. H., McCluskey, J. T., Lindsay, J. R., Ennis, C. N., Gamble, R., Sheridan, B., Barnett, C. R., McNulty, H., Bell, P. M., & Flatt, P. R. (2003). Demonstration of glycated insulin in human diabetic plasma and decreased biological activity assessed by euglycemic-hyperinsulinemic clamp technique in humans. Diabetes, 52, 492–498.
Wu, L., & Juurlink, B. H. J. (2002). Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension, 39, 809–814.
Miyata, T., Hori, O., Zhang, J., Yan, S. D., Ferran, L., Iida, Y., & Schmidt, A. M. (1996). The receptor for advanced glycation end products (RAGE) is a central mediator of the interaction of AGE-β2microglobulin with human mononuclear phagocytes via an oxidant-sensitive pathway. Implications for the pathogenesis of dialysis-related amyloidosis. Journal of Clinical Investigation, 98, 1088–1094.
Hangaishi, M., Taguchi, J., Miyata, T., Ikari, Y., Togo, M., Hashimoto, Y., Watanabe, T., Kimura, S., Kurokawa, K., & Ohno, M. (1998). Increased aggregation of human platelets produced by advanced glycation end products in vitro. Biochemical and Biophysical Research Communications, 248, 285–292.
Chakravarti, R. N., Kirshenbaum, L. A., & Singal, P. K. (1991). Atherosclerosis: Its pathophysiology with special reference to lipid peroxidation. Journal of Applied Cardiology, 6, 91–112.
Dhalla, N. S., Temsah, R. M., & Netticadan, T. (2000). Role of oxidative stress in cardiovascular diseases. Journal of Hypertension, 18, 655–673.
Bergamini, C. M., Gambetti, S., Dondi, A., & Cervellati, C. (2004). Oxygen, reactive oxygen species and tissue damage. Current Pharmaceutical Design, 10, 1611–1626.
Vasdev, S., Gill, V., & Singal, P. K. (2006). Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Experimental and Clinical Cardiology, 11, 206–216.
O’Brien, P. J., Siraki, A. G., & Shangari, N. (2005). Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Critical Reviews in Toxicology, 35, 609–662.
Voziyan, P. A., Metz, T. O., Baynes, J. W., & Hudson, B. G. (2002). A post-amadori inhibitor pridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. Journal of Biological Chemistry, 277, 3397–3403.
Cooper, M. E. (2004). The role of the rennin–angiotensin–aldosterone system in diabetes and its vascular complications. American Journal of Hypertension, 17, 16S–20S.
Mehta, P. K., & Griendling, K. K. (2007). Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. American Journal of Physiology Cell Physiology, 292, C82–C97.
Webber, M. A. (1999). Interrupting the rennin–angiotensin system: The role of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists in the treatment of hypertension. American Journal of Hypertension, 12, 189S–194S.
Duprez, D. A. (2006). Role of the rennin–angiotensin–aldosterone system in vascular remodeling and inflammation: A clinical review. Journal of Hypertension, 24, 983–991.
Thomas, M. C., Tikellis, C., Burns, W. M., Bialkowski, K., Cao, Z., Coughlan, M. T., Jandeleit-Dahm, K., Cooper, M. E., & Forbes, J. M. (2005). Interactions between renin angiotensin system and advanced glycation in the kidney. Journal of the American Society of Nephrology, 16, 2976–2984.
Fukami, K., Ueda, S., Yamagishi, S., Kato, S., Inagaki, Y., Takeuchi, M., Motomiya, Y., Bucala, R., Iida, S., Tamaki, K., Imaizumi, T., Cooper, M. E., & Okuda, S. (2004). AGEs activate mesangial TGF-β-Smad signaling via an angiotensin II type 1 receptor interaction. Kidney International, 66, 2137–2147.
Ikemoto, F., Song, G. B., Tominaga, M., & Yamamoto, K. (1988). Oxidation-induced increase in activity of angiotensin converting enzyme in the rat kidney. Biochemical and Biophysical Research Communications, 153, 1032–1037.
Koka, V., Wang, W., Huang, X. R., Kim-Mitsuyama, S., Truong, L. D., & Lan, H. Y. (2006). Advanced glycation end products activate a chymase-dependent angiotensin II-generating pathway in diabetic complications. Circulation, 113, 1353–1360.
Huang, X. R., Chen, W. Y., Truong, L. D., & Lan, H. Y. (2003). Chymase is upregulated in diabetic nephropathy: Implications for an alternate pathway of angiotensin II-mediated diabetic renal and vascular disease. Journal of the American Society of Nephrology, 14, 1738–1747.
Schupp, N., Schinzel, R., Heidland, A., & Stopper, H. (2005). Genotoxicity of advanced glycation end products: Involvement of oxidative stress and of angiotensin II type 1 receptors. Annals of the New York Academy of Sciences, 1043, 685–695.
Bohlender, J., Franke, S., Sommer, M., & Stein, G. (2005). Advanced glycation end products: A possible link to angiotensin in an animal model. Annals of the New York Academy of Sciences, 1043, 681–684.
Nakamura, K., Yamagishi, S., Nakamura, Y., Takenaka, K., Matsui, T., Jinnouchi, Y., Imaizumi, T. (2005). Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvascular Research, 70, 137–141.
Forbes, J. M., Thorpe, S. R., Thallas-Bonke, V., Pete, J., Thomas, M. C., Deemer, E. R., Bassal, S., El-Osta, A., Long, D. M., Panagiotopoulos, S., Jerums, G., Osicka, T. M., & Cooper, M. E. (2005). Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. Journal of the American Society of Nephrology, 16, 2363–2372.
Nangaku, M., Miyata, T., Sada, T., Mizuno, M., Inagi, R., Ueda, Y., Ishikawa, N., Yuzawa, H., Koike, H., Van Ypersele de Strihou, C., & Kurokawa, K. (2003). Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a Type 2 diabetic nephropathy rat model. Journal of the American Society of Nephrology, 14, 1212–1222.
Coughlan, M. T., Thallas-Bonke, V., Pete, J., Long, D. M., Gasser, A., Tong, D. C. K., Arnstein, M., Thorpe, S. R., Cooper, M. E., & Forbes, J. M. (2007). Combination therapy with the advanced glycation end product cross-link breaker, Alagebrium, and angiotensin converting enzyme inhibitors in diabetes: Synergy or redundancy? Endocrinology, 148, 886–895.
Davis, B. J., Forbes, J. M., Thomas, M. C., Jerums, G., Burns, W. C., Kawachi, H., Allen, T. J., & Cooper, M. E. (2004). Superior renoprotective effects of combination therapy with ACE and AGE inhibition in the diabetic spontaneously hypertensive rat. Diabetologia, 47, 89–97.
Aoki, K., Kawaguchi, Y., Sato, K., Kondo, S., & Yamamoto, M. (1982). Clinical and pharmacological properties of calcium antagonists in essential hypertension in humans and spontaneously hypertensive rats. Journal of Cardiovascular Pharmacology, 4, S298–S302.
Robinson, B. F. (1984). Altered calcium handling as a cause of primary hypertension. Journal of Hypertension, 2, 453–460.
Ding, Y. A. (1996). Thrombogenic and lipid risk factors in hypertension and coronary artery disease. Japanese Circulation Journal, 60, 75–84.
Bidasee, K. R., Nallani, K., Yu, Y., Cocklin, R. R., Zhang, Y., Wang, M., Dincer, U. D., & Besch, H. R. Jr. (2003). Chronic diabetes increases advanced glycation end products on cardiac ryanodine receptors/calcium-release channels. Diabetes, 52, 1825–1836.
Chang, T., Wang, R., & Wu, L. (2005). Methylglyoxal-induced nitric oxide and peroxynitrite production in vascular smooth muscle cells. Free Radical Biology and Medicine, 38, 286–293.
Viner, R. I., Williams, T. D., & Schoneich, C. (1999). Peroxynitrite modification of protein thiols: Oxidation, nitrosylation, and S-glutathione of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry, 38, 12408–12415.
Zhou, Q. G., Liu, N. F., & Xie, P. L. (1997). Expression of receptor for advanced glycosylation end products (AGEP) and inhibition of AGEP-induced cytosolic calcium elevation by diltiazem in cultured rat aortic smooth muscle cells. Zhongguo Yao Li Xue Bao, 8, 425–430.
Cook, L. J., Davies, J., Yates, A. P., Elliott, A. C., Lovell, J., Joule, J. A., Pemberton, P., Thornalley, P. J., & Best, L. (1998). Effects of methylglyoxal on rat pancreatic β-cells. Biochemical Pharmacology, 55, 1361–1367.
Taddei, S., Ghiadoni, L., Virdis, D., & Salvetti, V. A. (2003). Mechanisms of endothelial dysfunction: Clinical significance and preventative non-pharmacological therapeutic strategies. Current Pharmaceutical Design, 9, 2385–2402.
Shinozaki, K., Kashiwagi, A., Masada, M., & Okamura, T. (2004). Molecular mechanisms of impaired endothelial function associated with insulin resistance. Current Drug Targets, 4, 1–11.
Palmer, R. M. J., Rees, D. D., Ashton, D. S., & Moncada, S. (1988). l-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochemical and Biophysical Research Communications, 153, 1251–1256.
Kahn, N. N., Acharya, K., Bhattacharya, S., Acharya, R., Mazumder, S., Bauman, W. A., & Sinha, A. K. (2000). Nitric oxide: The “second messenger” of insulin. IUBMB Life, 49, 441–450.
Alp, N. J., & Channon, K. M. (2004). Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arteriosclerosis Thrombosis and Vascular Biology, 24, 413–420.
Lo, T. W. C., Westwood, M. E., McLellan, A. C., Selwood, T., & Thornalley, P. J. (1994). Binding and modification of proteins by methylglyoxal under physiological conditions. Journal of Biological Chemistry, 269, 32299–32305.
Odani, H., Iijima, K., Nakata, M., Miyata, S., Kusunoki, H., Yasuda, Y., Hiki, Y., Irie, S., Maeda, K., & Fujimoto, D. (2001). Identification of Nω-carboxymethylarginine, a new advanced glycation endproduct in serum proteins of diabetic patients: Possibility of a new marker of aging and diabetes. Biochemical and Biophysical Research Communications, 285, 1232–1236.
Forstermann, U., & Munzel, T. (2006). Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation, 113, 1708–1714.
Ishii, M., Shimizu, S., Nagai, T., Shiota, K., Kiuchi, Y., & Yamamoto, T. (2001). Stimulation of tetrahydrobiopterin synthesis induced by insulin: Possible involvement of phosphtidylinositol 3-kinase. International Journal of Biochemistry & Cell Biology, 33, 65–73.
Scharfstein, J. S., Keaney, J. F. Jr., Slivka, A., Welch, G. N., Vita, J. A., Stamier, J. S., & Loscalzo, J. (1994). In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. Journal of Clinical Investigation, 94, 1432–1439.
Alencar, J. L., Lobysheva, I., Geffard, M., Sarr, M., Schott, C., Schini-Kerth, V. B., Nepveu, F., Stoclet, J. C., & Muller, B. (2003). Role of S-nitrosation of cysteine residues in long-lasting inhibitory effect of nitric oxide. Molecular Pharmacology, 63, 1148–1158.
Farkas, J., & Menzel, E. J. (1995). Proteins lose their nitric oxide stabilizing function after advanced glycosylation. Biochimica et Biophysica Acta, 1245, 305–310.
Selwood, T., & Thornalley, P. J. (1993). Binding of methylglyoxal to albumin and formation of fluorescent adducts. Inhibition by arginine, N-acetylarginine and aminoguanidine. Biochemical Society Transactions, 21, 170S.
Iwashima, Y., Eto, M., Hata, A., Kaku, K., Horuichi, S., Ushikubi, F., & Sano, H. (2000). Advanced glycation end products-induced gene expression of scavenger receptors in cultured human monocyte-derived macrophages. Biochemical and Biophysical Research Communications, 277, 368–380.
Quehenberger, P., Bierhaus, A., Fasching, P., Muellner, C., Klevesath, M., Hong, M., Stier, G., Satter, M., Schleicher, E., Speiser, W., & Nawroth, P. P. (2000). Endothelin 1 transcription is controlled by nuclear factor-κB in AGE-stimulated cultured endothelial cells. Diabetes, 49, 1561–1570.
Nevado, J., Peiro, C., Vallejo, S., El-Assar, M., Lafuente, N., Matesanz, N., Azcutia, V., Cercas, E., Sanchez-Ferrer, C. F., & Rodrigues-Manas, L. (2005). Amadori adducts activate nuclear factor-κ-B-related proinflammatory genes in cultured human peritoneal mesothelial cells. British Journal of Clinical Pharmacology, 146, 268–279.
Wang, Y., Marshall, S. M., Thompson, M. G., & Hoenich, N. A. (2005). Cardiovascular risk in patients with end-stage renal disease: A potential role for advanced glycation end products. Contributions to Nephrology, 149, 168–174.
Brown, B. E., Dean, R. T., & Davies, M. J. (2005). Glycation of low-density lipoproteins by methylglyoxal and glycoaldehyde gives rise to the in vitro formation of lipid-laden cells. Diabetologia, 48, 361–369.
Knott, H. M., Brown, B. E., Davies, M. J., & Dean, R. T. (2003). Glycation and glycoxidation of low-density lipoproteins by glucose and low-molecular mass aldehydes. European Journal of Biochemistry, 270, 3572–3582.
Napoli, C., Triggiani, M., Palumbo, G., Condorelli, M., Chiariello, M., & Ambrosio, G. (1997). Glycosylation enhances oxygen radical-induced modifications and decreases acetylhydrolase activity of human low density lipoprotein. Basic Research in Cardiology, 92, 96–105.
Sasaki, J., & Cottam, G. L. (1982). Glycosylation of LDL decreases its ability to interact with high affinity receptors of human fibroblasts in vitro and decreases its clearance from rabbit plasma in vivo. Biochimica et Biophysica Acta, 713, 199–207.
Makita, T., Tanaka, A., & Numano, F. (1999). Effect of glycated low density lipoprotein on smooth muscle cell proliferation. International Journal of Angiology, 18, 331–334.
Napoli, C., Lerman, L. O., de Nigris, F., Loscalzo, J., & Ignarro, L. J. (2002). Glycoxidized low-density lipoprotein downregulates endothelial nitric oxide synthase in human coronary cells. Journal of the American College of Cardiology, 40, 1515–1522.
Chang, P. C., Chen, T. H., Chang, C. J., Hou, C. C., Chan, P., & Lee, H. M. (2004). Advanced glycosylation end products induce inducible nitric oxide synthase (iNOS) expression via p38 MAPK-dependent pathway. Kidney International, 65, 1664–1675.
Hedrick, C. C., Thorpe, S. R., Fu, M. X., Harper, C. M., Yoo, J., Kim, S. M., Wong, H., & Peters, A. L. (2000). Glycation impairs high-density lipoprotein function. Diabetologia, 43, 312–320.
Ferretti, G., Bacchetti, T., Marchionni, C., Calderelli, L., & Curatola, G. (2001). Effect of glycation of high density lipoproteins on their physiochemical properties and on paraoxonase activity. Acta Diabetologia, 38, 163–169.
Packer, L., Roy, S., & Sen, C. K. (1997). α-Lipoic acid: A metabolic antioxidant and potential redox modulator of transcription. Advances in Pharmacology, 38, 79–101.
Mizutani, K., Ikeda, K., & Yamori, Y. (2000). Resveratrol inhibits AGEs-induced proliferation and collagen synthesis activity in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Biochemical and Biophysical Research Communications, 274, 61–67.
Huang, S. M., Wu, C. H., & Yen, G. C. (2006). Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Molecular Nutrition & Food Research, 50, 1129–1139.
Yi, X., & Maeda, N. (2006). α-Lipoic acid prevents the increase in atherosclerosis induced by diabetes in apolipoprotein E-deficient mice fed high-fat/low-cholesterol diet. Diabetes, 55, 2238–2244.
Foster, T. S. (2007). Efficacy and safety of α-lipoic acid supplementation in the treatment of symptomatic diabetic neuropathy. The Diabetes Educator, 33, 111–117.
Sola, S., Mir, M. Q. S., Cheema, F. A., Khan-Merchant, N., Menon, R. G., Parthasarathy, S., & Khan, B. V. (2005). Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome. Results of the Irbesartan and lipoic acid in endothelial dysfunction (ISLAND) study. Circulation, 111, 343–348.
Wang, X. L., Rainwater, D. L., Mahaney, M. C., & Stocker, R. (2004). Cosupplementation with vitamin E, & Coenzyme Q10 reduces circulating markers of imflammation in baboons. The American Journal of Clinical Nutrition, 80, 649–655.
Riccioni, G., Bucciarelli, T., Mancini, B., Iilo, C., Capra, V., & D’Orazio, N. (2007). The role of the antioxidant vitamin supplementation in the prevention of cardiovascular disease. Expert Opinion on Investigational Drugs, 16, 25–32.
Forbes, J. M., Yee, L. T., Thallas, V., Lassila, M., Candido, R., Jandeleit-Dahm, K. A., Thomas, M. C., Burns, W. C., Deemer, E. K., Thorpe, S. R., Cooper, M. E., & Allen, T. J. (2004). Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes, 53, 1813–1823.
Figarola, J. L., Scott, S., Loera, S., Xi, B., Synold, T., & Rahbar, S. (2002). Renoprotective and lipid-lowering effects of LR compounds, novel advanced glycation end product inhibitors, in streptozotocin-induced diabetic rats. Annals of the New York Academy of Sciences, 1043, 767–776.
McGill, J. B., Degenhardt, T. P., Szabo, J. R., Khalifah, R. G., & Schotzinger, R. J. (2004). A phase 2 clinical investigation of Pyridoxamine (Pyridorin™) in Type 1 and Type 2 diabetic patients with overt diabetic nephropathy (PYR-205/207) Am. Diabetes Assoc., 64th annual conference, Abstract no. 581-P. http://scientificsessions.diabetes.org/Abstracts/index.cfm?fuseaction=Locator.PreviewAbstract&popup=yes&NoLayout=Yes&AbstractID=6024 (accessed February 19, 2007).
Bell, D. S. H., Degenhardt, T. P., Szabo, J. R., Khalifah, R. G., & Schotzinger, R. J. (2004). Investigation of the safety and efficacy of pyridoxamine (Pyridorin™) in patients with diabetic nephropathy (PYR-206) American Diabetes Association, 64th annual conference, Abstract no. 504-P. http://scientificsessions.diabetes.org/Abstracts/index.cfm?fuseaction=Locator.PreviewAbstract&popup=yes&NoLayout=Yes&AbstractID=6281 (accessed February 19, 2007).
Miyata, T., Van Ypersele de Strihou, C., Ueda, Y., Ichimori, K., Inagi, R., Onogi, H., Ishikawa, N., Nangaku, M., & Kurokawa, K. (2002). Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: Biochemical mechanisms. Journal of the American Society of Nephrology, 13, 2478–2487.
Akira, K., Amano, M., Okajima, F., Hashimoto, T., & Oikawa, S. (2006). Inhibitory effects of Amlodipine and Fluvastatin on the deposition of advanced glycation end products in aortic wall of cholesterol and fructose-fed rabbits. Biological & Pharmaceutical Bulletin, 29, 75–81.
Beisswenger, P. J., Howell, S. K., Touchette, A. D., Lal, S., & Szwergold, B. S. (1999). Metformin reduces systemic methylglyoxal levels in Type 2 diabetes. Diabetes, 48, 198–202.
Acknowledgement
We would like to thank the Canadian Institutes of Health Research Regional Partnership Program for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasdev, S., Gill, V. & Singal, P. Role of Advanced Glycation End Products in Hypertension and Atherosclerosis: Therapeutic Implications. Cell Biochem Biophys 49, 48–63 (2007). https://doi.org/10.1007/s12013-007-0039-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-007-0039-0