Abstract
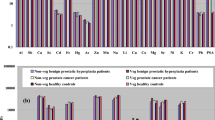
Prostate cancer is known to be affected by the heavy metal levels and oxidative damage of the body, yet there are very few studies which look into the way it occurs. The aim of this study was to determine whether blood and tissue lead (Pb), cadmium (Cd), and selenium (Se) levels are associated with oxidative damage in the context of prostate cancer progression and development. Seventy-nine patients comprising 25 patients with benign prostatic hypertrophy (BPH), 23 patients with malignant prostatic carcinoma (malign Ca), 16 patients with low-grade prostatic intraepithelial neoplasia (LGPIN), and 15 patients with high-grade prostatic intraepithelial neoplasia (HGPIN) diagnosed on the basis of their clinical profile, transrectal ultrasonography, and histopathology were included in this study. Cd and Pb levels in whole blood were found to be increased in patients with HGPIN compared with the BPH group; also, the levels of Cd in whole blood and tissue were found to be increasing in patients with malign Ca, unlike BPH patients. Moreover, the levels of malondialdehyde (MDA) in plasma and tissue were significantly increased in malign Ca, LGPIN, and HGPIN than those in BPH. However, the levels of tissue Pb were found to be decreasing in BPH, unlike the malign Ca and HGPIN patients, and the levels of tissue protein carbonyls in malign Ca were significantly lower than those in HGPIN. The levels of tissue reduced glutathione (GSH) in malign Ca were significantly lower than those in BPH. Additionally, the levels of Se in serum and tissue in LGPIN were significantly lower than those in BPH. The serum Se levels in HGPIN were also significantly lower than those in BPH and malign Ca groups. Furthermore, the concentrations of serum Se in LGPIN were significantly lower than those in malign Ca. From the Pearson correlation analysis, there were significant positive correlations between tissue Cd and MDA levels in malign Ca, LGPIN, and HGPIN and between the tissue Pb and tissue MDA and protein carbonyl levels in malign Ca. Blood Pb and tissue Pb were also significantly positively correlated with plasma MDA and protein carbonyl levels in malign Ca. In addition, blood Pb was significantly positively correlated with tissue MDA and protein carbonyl levels in malign Ca, and a significant positive correlation was also found between blood Cd and plasma protein carbonyls and tissue MDA in LGPIN. We observed that altered prooxidant–antioxidant balance and heavy metal levels may lead to an increase in oxidative damage and may consequently play an important role in prostate carcinogenesis. These findings indicate that changes in the levels of Pb, Cd, Se, MDA, protein carbonyls, and GSH in the blood and/or tissue are related to the prostatic carcinoma development and progression, although triggering one of the mentioned changes is unknown; therefore, further study is required to determine the exact steps of the process and clarify the roles of different substances in order to obtain a more detailed explanation of the phenomenon.






Similar content being viewed by others
References
Fenech M, Ferguson LR (2001) Vitamins/minerals and genomic stability in humans. Mutat Res 475:1–6
Burcham PC (1999) Internal hazards: baseline DNA damage by endogenous products of normal metabolism. Mutat Res 443:11–36
Gopalkrishnan K (1998) Characteristics of semen parameters in a selected population of Indian men over a period of 10 years. Curr Sci 75:939–942
Stohs SJ, Baggihi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Med 18:321–336
Forsberg L, de Faire U, Morgenstern R (2001) Oxidative stress, human genetic variation, and disease. Arch Biochem Biophys 389:84–93
Waalkers MP (2003) Cadmium carcinogenesis. Mutat Res 533:107–120
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences. Biochimie 88:1549–1559
Siddiqui MK, Jyoti SS, Mehrotra PK, Singh K, Sarangi R (2006) Comparison of some trace elements concentration in blood, tumor free breast and tumor tissues of women with benign and malignant breast lesions: an Indian study. Environ Int 32:630–637
Kiziler AR, Aydemir B, Onaran I, Alici B, Ozkara H, Gulyasar T, Akyolcu MC (2007) High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol Trace Elem Res 120:82–91
Kiziler AR, Aydemir B, Guzel S, Alici B, Ataus S, Tuna MB, Durak H (2010) May the level and ratio changes of trace elements be utilized in identification of disease progression and grade in prostatic cancer? Trace Elem Elect 2:65–72
Aydin A, Arsova-Sarafinovska Z, Sayal A, Eken A, Erdem O, Erten K, Ozgok Y, Dimovski A (2006) Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem 39:176–179
Guntupalli JNR, Padala S, Gummuluri AVRM, Muktineni RK, Byreddy SR, Sreerama L, Kedarisetti PC, Angalakuduru DP, Satti BR, Venkatathri V, Pullela VBRL, Gavarasana S (2007) Trace elemental analysis of normal, benign hypertrophic and cancerous tissues of the prostate gland using the particle-induced X-ray emission technique. European J Cancer Prevent 16:108–115
Feustel A, Wennrich R (1986) Zinc and cadmium plasma and erythrocyte levels in prostatic carcinoma, BPH, urological malignancies, and inflammations. Prostate 8:75–79
Schöpfer J, Drasch G, Schrauzer GN (2010) Selenium and cadmium levels and ratios in prostates, livers, and kidneys of nonsmokers and smokers. Biol Trace Elem Res 134:180–187
Golovine K, Makhov P, Uzzo RG, Kutikov A, Kaplan DJ, Fox E, Kolenko VM (2010) Cadmium down-regulates expression of XIAP at the post-transcriptional level in prostate cancer cells through an NF-kappaB-independent, proteasome-mediated mechanism. Mol Cancer 9:183
Gleason DF (2004) Histologic grading and clinical staging of prostatic carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds) Pathology and genetics of tumours of the urinary system and male genital organs. WHO classification of tumours. IARC, Lyon, pp 167–214
Buege JA, Aust STD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Anderson ME (1985) Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol 113:548–555
Levine RL, Wehr N, Williams JA, Stadtman ER, Shacter E (2000) Determination of carbonyl groups in oxidized proteins. Methods Mol Biol 99:15–24
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:269–275
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals, and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Aymelek G, Erten D, Aslan S, Akinci M, Simşek B, Torun M (2006) Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int 30:376–380
Nishikawa M (2008) Reactive oxygen species in tumor metastasis. Cancer Lett 266:53–59
Drasch G, Schöpfer J, Schrauzer GN (2005) Selenium/cadmium ratios in human prostates: indicators of prostate cancer risk of smokers and nonsmokers, and relevance to the cancer protective effects of selenium. Biol Trace Elem Res 103:103–107
Witkiewicz-Kucharczyk A, Bal W (2006) Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol Lett 162:29–42
Bounous G, Beer D (2004) Molecular pathogenesis and prevention of prostate cancer. Anticancer Res 24:553–554
Henkler F, Brinkmann J, Luch A (2010) The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers 2:376–396
Huang YL, Sheu JY, Lin TS (1999) Association between oxidative stress and changes of trace elements in patients with breast cancer. Clin Biochem 32:131–136
Badjatia N, Satyam A, Singh P, Seth A, Sharma A (2010) Altered antioxidant status and lipid peroxidation in Indian patients with urothelial bladder carcinoma. Urol Oncol 28:360–367
Sharma A, Rajjapa M, Saxena A, Sharma M (2007) Antioxidant status in advanced cervical cancer patients undergoing neoadjuvant chemoradiation. Br J Biomed Sci 64:23–27
Zarros A, Skandali N, Al-Humadi H, Liapi C (2008) Cadmium (Cd) as a carcinogenetic factor and its participation in the induction of lung cancer. Pneumon 21:172–177
Schrauzer GN (2000) Anticarcinogenic effects of selenium. Cell Mol Life Sci 57:1864–1873
van Wijngaarden E, Singer EA, Palapattu GS (2008) Prostate-specific antigen levels in relation to cadmium exposure and zinc intake: results from the 2001–2002 National Health and Nutrition Examination Survey. Prostate 68:122–128
Arsova-Sarafinovska Z, Eken A, Matevska N, Erdem O, Sayal A, Savaser A, Banev S, Petrovski D, Dzikova S, Georgiev V, Sikole A, Ozgök Y, Suturkova L, Dimovski AJ, Aydin A (2009) Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin Biochem 42:1228–1235
Biri H, Oztürk HS, Kaçmaz M, Karaca K, Tokuçoğlu H, Durak I (1999) Activities of DNA turnover and free radical metabolizing enzymes in cancerous human prostate tissue. Cancer Invest 17:314–319
Yılmaz MI, SaglamK SA, Gok DE, Basal S, Kilic S, Akay C, Kocar IH (2004) Antioxidant system activation in prostate cancer. Biol Trace Elem Res 98:13–19
Pace G, Di Massimo C, De Amicis D, Corbacelli C, Di Renzo L, Vicentini C, Miano L, Tozzi Ciancarelli MG (2010) Oxidative stress in benign prostatic hyperplasia and prostate cancer. Urol Int 85:328–333
Ozmen H, Erulas FA, Karatas F, Cukurovali A, Yalcin O (2006) Comparison of the concentration of trace metals (Ni, Zn, Co, Cu and Se), Fe, vitanmins A, C and E, and lipid peroxidation in patients with prostate cancer. Clin Chem Lab Med 44:175–179
Almushatat AS, Talwar D, McArdle PA, Williamson C, Sattar N, O’Reilly DS, Underwood MA, McMillan DC (2006) Vitamin antioxidants, lipid peroxidation and the systemic inflammatory response in patients with prostate cancer. Int J Cancer 118:1051–1053
Doğru-Abbasoğlu S, Aykaç-Toker G, Koçak T, Unlüer E, Uysal M (1999) Antioxidant enzyme activities and lipid peroxides in the plasma of patients with benign prostatic hyperplasia or prostate cancer are not predictive. J Cancer Res Clin Oncol 125:402–404
Hoque A, Ambrosone CB, Till C, Goodman PJ, Tangen C, Kristal A, Lucia S, Wang Q, Kappil M, Thompson I, Hsing AW, Parnes H, Santella RM (2010) Serum oxidized protein and prostate cancer risk within the prostate cancer prevention trial. Cancer Prev Res (Phila) 3:478–483
Akiibinu MO, Ogundahunsi AO, Kareem OI, Adesiyan AA, Idonije BO, Adeniyi FAA (2011) Trace metals and oxidative metabolic changes in malignant prostate cancer patients. Afr J Biochem Res 5:102–105
Blum J, Fridovich I (1985) Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys 240:500–508
Surapaneni KM, Venkata GR (2006) Lipid peroxidatin and antioxidant status in patients with carcinoma of prostate. Indian J Physiol Pharmacol 50:350–354
Hietanen E, Bartsch H, Bereziat JC, Camus AM, McClinton S, Eremin O, Davidson L, Boyle P (1994) Diet and oxidative stress in breast, colon and prostate cancer patients: a case–control study. Eur J Clin Nutr 48:575–586
Jung K, Seydel B, Rudolph B, Lein M, Cronauer MV, Henke W, Hampel G, Schnorr D, Loening SA (1997) Antioxidant enzymes in malignant prostate cell lines and in primary cultured prostatic cells. Free Radic Biol Med 23:127–133
Pastore A, Federici G, Bertini E, Piemonte F (2003) Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333:19–39
Ahmed MI, Fayed ST, Hossein H, Tash FM (1999) Lipid peroxidation and antioxidant status in human cervical carcinoma. Dis Markers 15:283–291
Liu X, Fu YM, Meadows GG (2011) Differential effects of specific amino acid restriction on glucose metabolism, reduction/oxidation status and mitochondrial damage in DU145 and PC3 prostate cancer cells. Oncol Lett 18:349–355
Lim HW, Hong S, Jin W, Lim S, Kim SJ, Kang HJ, Park EH, Ahn K, Lim CJ (2005) Up-regulation of defense enzymes is responsible for low reactive oxygen species in malignant prostate cancer cells. Exp Mol Med 37:497–506
Gupta A, Butts B, Kwei KA, Dvorakova K, Stratton SP, Briehl MM, Bowden GT (2001) Attenuation of catalase activity in the malignant phenotype plays a functional role in an in vitro model for tumor progression. Cancer Lett 28:115–125
Cobanoglu U, Demir H, Sayir F, Duran M, Mergan D (2010) Some mineral, trace element and heavy metal concentrations in lung cancer. Asian Pac J Cancer Prev 11:1383–1388
Acknowledgments
This work was supported by The Research Fund of Istanbul University (Project BYP-2621/30062008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzel, S., Kiziler, L., Aydemir, B. et al. Association of Pb, Cd, and Se Concentrations and Oxidative Damage-Related Markers in Different Grades of Prostate Carcinoma. Biol Trace Elem Res 145, 23–32 (2012). https://doi.org/10.1007/s12011-011-9162-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9162-2