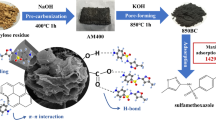
Abstract
Purpose
Sulfamethazine (SMT) is increasingly detected in environmental matrices due to its versatile use as antibiotics. We aimed to investigate the benefits and roles of steam activation of biochars with respect to SMT sorption kinetics and equilibrium sorption.
Materials and methods
Biochars were produced from burcucumber plant and tea waste using a pyrolyzer at a temperature of 700 °C for 2 h. The biochar samples were treated with 5 mL min−1 of steam for an additional 45 min for post-synthesis steam activation. The SMT sorption on the unmodified and steam activated biochars were compared.
Results and discussion
The time taken to reach equilibrium was significantly less for steam activated biochars (∼4 h) than non-activated biochars (>24 h). Up to 98 % of SMT could be removed from aqueous solutions by steam activated biochars. The sorption kinetic behaviors were well described by the pseudo-second model and SMT sorption rates of steam activated biochars (k 2 ∼ 1.11–1.57 mg g−1 min−1) were significantly higher than that of the unmodified biochars (k 2 ∼ 0.04–0.11 mg g−1 min−1) because of increased availability of accessible porous structure with averagely larger pore diameters. Moreover, the equilibrium sorption on the unmodified biochars was significantly influenced by increasing solution pH (∼30–50 % reduction) because of speciation change of SMT, whereas steam activated biochars manifested much stronger sorption resilience against pH variation (∼2–4 % reduction only) because the enhanced porosity offset the effect of unfavorable electrostatic repulsion.
Conclusions
The observed features of steam activated biochars would render their applications more versatile and reliable in field throughout changeable environmental conditions.


Similar content being viewed by others
References
Ahmad M, Lee SS, Dou X, Mohan D, Sung JK, Yang JE, Ok YS (2012) Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour Technol 118:536–544
Ahmad M, Rajapaksha AU, Lim JE, Zhang M, Bolan N, Mohan D, Vithanage M, Lee SS, Ok YS (2014) Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 99:19–23
Aust MO, Thiele-Bruhn S, Seeger J, Godlinski F, Meissner R, Leinweber P (2010) Sulfonamides leach from sandy loam soils under common agricultural practice. Water Air Soil Pollut 211:143–156
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47–52
Benjamin MM (2002) Adsorption reactions. Water chemistry. McGraw-Hill, New York, pp 550–627
Boxall ABA, Fogg LA, Blackwell PA, Blackwell P, Kay P, Pemberton EJ, Croxford A (2004) Veterinary medicines in the environment. Rev Environ Contam Toxicol, Springer, New York, pp 1–91
Chia CH, Downie A, Munroe P (2015) Characteristics of biochar: physical and structural properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management. Earthscan, London, pp 89–111
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrolysis 72:243–248
Frohne T, Diaz-Bone RA, Du Laing G, Rinklebe J (2015) Impact of systematic change of redox potential on the leaching of Ba, Cr, Sr, and V from a riverine soil into water. J Soils Sediments 15:623–633
Guo X, Yang C, Dang Z, Zhang Q, Li Y, Meng Q (2013) Sorption thermodynamics and kinetics properties of tylosin and sulfamethazine on goethite. Chem Eng J 223:59–67
Haller MY, Müller SR, McArdell CS, Alder AC, Suter MJF (2002) Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography–mass spectrometry. J Chromatogr A 952:111–120
Hamscher G, Sczesny S, Höper H, Nau H (2002) Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Anal Chem 74:1509–1518
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper (II) on to peat. Adsorpt Sci Technol 20:797–815
Kim KR, Owens G, Kwon SI, So KH, Lee DB, Ok YS (2011) Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut 214:163–174
Lagergren S (1898) Zurtheorie der sogenannten adsorption gelosterstoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Lehmann J, Joseph S (2009) Biochar for environmental management: science and technology. Earthscan, London
Lim JE, Rajapaksha AU, Jeong SH, Kim SC, Kim KH, Lee SS, Ok YS (2014) Monitoring of selected veterinary antibiotics in animal carcass disposal site and adjacent agricultural soil. J Appl Biol Chem 57:189–196
Lima IM, Boateng AA, Klasson KT (2010) Physicochemical and adsorptive properties of fast-pyrolysis bio-chars and their steam activated counterparts. J Chem Technol Biotechnol 85:1515–1521
Liu MY, Tsang DCW, Hu J, Ng KTW, Liu T, Lo IMC (2008) Adsorption of methylene blue and phenol by wood waste derived activated carbon. J Environ Eng 134:338–345
Lussier MG, Zhang Z, Miller DJ (1998) Characterizing rate inhibition in steam/hydrogen gasification via analysis of adsorbed hydrogen. Carbon 36:1361–1369
Manyà JJ (2012) Pyrolysis for biochar purposes: a review to establish current knowledge gaps and research needs. Environ Sci Technol 46:7939–7954
Margalida A, Bogliani G, Bowden CGR, Donázar JA, Genero F, Gilbert M, Karesh WB, Kock R, Lubroth J, Manteca X, Naidoo V, Neimanis A, Sánchez-Zapata JA, Taggart MA, Vaarten J, Yon L, Kuiken T, Green RE (2014) One health approach to use of veterinary pharmaceuticals. Science 346:1296–1298
Mohan D, Sarswat A, Ok YS, Pittman CU Jr (2014) Organic and inorganic contaminants removal from water with biochar: a renewable, low cost and sustainable adsorbent—a critical review. Bioresour Technol 160:191–202
Novak JM, Lima I, Xing B, Gaskin JW, Steiner C, Das KC, Ahmedna M, Rehrah D, Watts DW, Busscher WJ, Harry S (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann Environ Sci 3:195–206
Ok YS, Kim SC, Kim KR, Lee SS, Moon DH, Lim KJ, Sung JK, Hur SO, Yang JE (2011) Monitoring of selected veterinary antibiotics in environmental compartments near a composting facility in Gangwon Province, Korea. Environ Monit Assess 174:693–701
Qiang Z, Adams C (2004) Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res 38:2874–2890
Rajapaksha AU, Vithanage M, Zhang M, Ahmad M, Mohan D, Chang SX, Ok YS (2014) Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour Technol 166:303–308
Rajapaksha AU, Vithanage M, Ahmad M, Seo DC, Cho JS, Lee SE, Lee SS, Ok YS (2015) Enhanced sulfamethazine removal by steam-activated invasive plant-derived biochar. J Hazard Mater 290:43–50
Rinklebe J, Shaheen SM, Frohne T (2015) Amendment of biochar reduces the release of toxic elements under dynamic redox conditions in a contaminated floodplain soil. Chemosphere 142:41–47
Sparks DL (1999) Soil physical chemistry, 2nd edn. CRC Press, New York
Sposito G (2004) The surface chemistry of natural particles. Oxford University Press, New York
Teixidó M, Pignatello JJ, Beltrán JL, Granados M, Peccia J (2011) Speciation of the ionizable antibiotic sulfamethazine on black carbon (biochar). Environ Sci Technol 45:10020–10027
Thiele-Bruhn S (2003) Pharmaceutical antibiotic compounds in soils—a review. J Plant Nutr Soil Sci 166:145–167
Tsang DCW, Yip ACK (2014) Comparing chemical-enhanced washing and waste-based stabilisation approach for soil remediation. J Soils Sediments 14:936–947
Tsang DCW, Hu J, Liu M, Zhang W, Lai KCK, Lo IMC (2007) Activated carbon produced from waste wood pallets: adsorption of three classes of dyes. Water Air Soil Pollut 184:141–155
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190:432–441
Vithanage M, Rajapaksha AU, Tang X, Thiele-Bruhn S, Kim KH, Lee SE, Ok YS (2014) Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived-biochar. J Environ Manag 141:95–103
Wang S, Gao B, Zimmerman AR, Li Y, Ma L, Harris WG, Migliaccio KW (2015) Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour Technol 175:391–395
Wu FC, Tseng RL, Juang RS (2009) Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem Eng J 150:366–373
Yang JF, Ying GG, Yang LH, Zhao JL, Liu F, Tao R, Yu ZQ (2009) Degradation behavior of sulfadiazine in soils under different conditions. J Environ Sci Health Part B 44:241–248
Zhang W, Zheng J, Zheng P, Tsang DCW, Qiu R (2015) Sludge-derived biochar for arsenic(III) immobilization: effects of solution chemistry on sorption behavior. J Environ Qual 44:1119–1126
Zhu X, Tsang DCW, Chen F, Li S, Yang X (2015) Ciprofloxacin adsorption on graphene and granular activated carbon: kinetics, isotherms, and effects of solution chemistry. Environ Technol 36:3094–3102
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF-2015R1A2A2A11001432).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Hailong Wang
Rights and permissions
About this article
Cite this article
Rajapaksha, A.U., Vithanage, M., Lee, S.S. et al. Steam activation of biochars facilitates kinetics and pH-resilience of sulfamethazine sorption. J Soils Sediments 16, 889–895 (2016). https://doi.org/10.1007/s11368-015-1325-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1325-x