Purpose
The aim of this study was to evaluate the pharmacokinetics of paclitaxel-loaded lipid nanocapsules (LNC) in rats to assess the intrinsic effect of the dosage form on the improvement of paclitaxel oral exposure.
Methods
Paclitaxel-loaded LNC were prepared and characterized in terms of size distribution, drug payload, and the kinetics of paclitaxel crystallization. Taxol®, Taxol® with verapamil, or paclitaxel-loaded LNC were administered orally to rats. The plasma concentration of paclitaxel was determined using liquid chromatography mass spectrometry.
Results
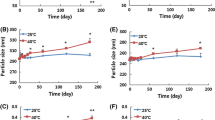
The average size of LNC was 60.9 ± 1.5 nm. The drug payload of paclitaxel was 1.91 ± 0.01 mg/g of aqueous dispersion. The encapsulation efficiency was 99.9 ± 1.0%, and 1.7 ± 0.1% of paclitaxel was crystallized after 24 h. The oral bioavailability of Taxol® alone was 6.5%. After oral administration of paclitaxel-loaded LNC or paclitaxel associated with verapamil, the area under the plasma concentration–time curve was significantly increased (about 3-fold) in comparison to the control group (p < 0.05).
Conclusions
The results indicated that LNC provided a promising new formulation to enhance the oral bioavailability of paclitaxel while avoiding the use of pharmacologically active P-gp inhibitors, such as verapamil.




Similar content being viewed by others
Abbreviations
- LNC:
-
lipid nanocapsules
- P-gp:
-
P-glycoprotein
References
E. K. Rowinsky (1993) ArticleTitleClinical pharmacology of Taxol J. Natl. Cancer Inst. Monogr. 15 25–37 Occurrence Handle7912527
E. K. Rowinsky R. C. Donehower (1995) ArticleTitlePaclitaxel (taxol) N. Engl. J. Med. 332 1004–1014 Occurrence Handle7885406 Occurrence Handle1:STN:280:ByqC1M7mtFQ%3D Occurrence Handle10.1056/NEJM199504133321507
K. C. Nicolaou W. M. Dai R. K. Guy (1994) ArticleTitleChemistry and biology of taxol Angew. Chem. Int. Ed. Engl. 33 15–44 Occurrence Handle10.1002/anie.199400151
A. K. Singla A. Garg D. Aggarwal (2002) ArticleTitlePaclitaxel and its formulations Int. J. Pharm. 235 179–192 Occurrence Handle11879753 Occurrence Handle1:CAS:528:DC%2BD38XhsV2qsro%3D Occurrence Handle10.1016/S0378-5173(01)00986-3
L. Zuylen Particlevan J. Verweij A. Sparreboom (2001) ArticleTitleRole of formulation vehicles in taxane pharmacology Invest. New Drugs 19 125–141 Occurrence Handle11392447 Occurrence Handle10.1023/A:1010618632738
S. A. Wissing O. Kayser R. H. Muller (2004) ArticleTitleSolid lipid nanoparticles for parenteral drug delivery Adv. Drug Deliv. Rev. 56 1257–1272 Occurrence Handle15109768 Occurrence Handle1:CAS:528:DC%2BD2cXjtl2ktrg%3D Occurrence Handle10.1016/j.addr.2003.12.002
D. Garrec ParticleLe S. Gori L. Luo D. Lessard D. C. Smith M. A. Yessine M. Ranger J. C. Leroux (2004) ArticleTitlePoly(N-vinylpyrrolidone)-block-poly(D,L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation J. Control. Release 99 83–101 Occurrence Handle15342183 Occurrence Handle10.1016/j.jconrel.2004.06.018
T. K. Yeh Z. Lu M. G. Wientjes J. L. Au (2005) ArticleTitleFormulating paclitaxel in nanoparticles alters its disposition Pharm. Res. 22 867–874 Occurrence Handle15948030 Occurrence Handle1:CAS:528:DC%2BD2MXltVKnurk%3D Occurrence Handle10.1007/s11095-005-4581-4
L. Mu S. S. Feng (2003) ArticleTitleA novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS J. Control. Release 86 33–48 Occurrence Handle12490371 Occurrence Handle1:CAS:528:DC%2BD38XpsFSjsro%3D Occurrence Handle10.1016/S0168-3659(02)00320-6
P. Gao B. D. Rush W. P. Pfund T. Huang J. M. Bauer W. Morozowich M. S. Kuo M. J. Hageman (2003) ArticleTitleDevelopment of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability J. Pharm. Sci. 92 2386–2398 Occurrence Handle14603484 Occurrence Handle1:CAS:528:DC%2BD3sXps1OisLY%3D Occurrence Handle10.1002/jps.10511
M. M. Malingre J. H. Beijnen J. H. Schellens (2001) ArticleTitleOral delivery of taxanes Invest. New Drugs 19 155–162 Occurrence Handle11392449 Occurrence Handle1:CAS:528:DC%2BD3MXktF2qurk%3D Occurrence Handle10.1023/A:1010635000879
J. Asperen Particlevan O. Tellingen Particlevan M. A. Valk Particlevan der M. Rozenhart J. H. Beijnen (1998) ArticleTitleEnhanced oral absorption and decreased elimination of paclitaxel in mice cotreated with cyclosporin A Clin. Cancer Res. 4 2293–2297 Occurrence Handle9796957
H. A. Bardelmeijer J. H. Beijnen K. R. Brouwer H. Rosing W. J. Nooijen J. H. Schellens O. Tellingen Particlevan (2000) ArticleTitleIncreased oral bioavailability of paclitaxel by GF120918 in mice through selective modulation of P-glycoprotein Clin. Cancer Res. 6 4416–4421 Occurrence Handle11106262 Occurrence Handle1:CAS:528:DC%2BD3cXovVSgu7Y%3D
J. S. Woo C. H. Lee C. K. Shim S. J. Hwang (2003) ArticleTitleEnhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031 Pharm. Res. 20 24–30 Occurrence Handle12608532 Occurrence Handle1:CAS:528:DC%2BD3sXoslKiug%3D%3D Occurrence Handle10.1023/A:1022286422439
M. Ramaswamy X. Zhang H. M. Burt K. M. Wasan (1997) ArticleTitleHuman plasma distribution of free paclitaxel and paclitaxel associated with diblock copolymers J. Pharm. Sci. 86 460–464 Occurrence Handle9109049 Occurrence Handle1:CAS:528:DyaK2sXhslamt7k%3D Occurrence Handle10.1021/js960333n
A. Sparreboom J. Asperen Particlevan U. Mayer A. H. Schinkel J. W. Smit D. K. Meijer P. Borst W. J. Nooijen J. H. Beijnen O. Tellingen Particlevan (1997) ArticleTitleLimited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine Proc. Natl. Acad. Sci. USA 94 2031–2035 Occurrence Handle9050899 Occurrence Handle1:CAS:528:DyaK2sXhslCjs7o%3D Occurrence Handle10.1073/pnas.94.5.2031
C. M. Kruijtzer J. H. Beijnen J. H. Schellens (2002) ArticleTitleImprovement of oral drug treatment by temporary inhibition of drug transporters and/or cytochrome P450 in the gastrointestinal tract and liver: an overview Oncologist 7 516–530 Occurrence Handle12490739 Occurrence Handle1:CAS:528:DC%2BD3sXksV2hug%3D%3D Occurrence Handle10.1634/theoncologist.7-6-516
J. Asperen Particlevan O. Tellingen Particlevan A. Sparreboom A. H. Schinkel P. Borst W. J. Nooijen J. H. Beijnen (1997) ArticleTitleEnhanced oral bioavailability of paclitaxel in mice treated with the P-glycoprotein blocker SDZ PSC 833 Br. J. Cancer 76 1181–1183 Occurrence Handle9365166
S. Yang R. N. Gursoy G. Lambert S. Benita (2004) ArticleTitleEnhanced oral absorption of paclitaxel in a novel self-microemulsifying drug delivery system with or without concomitant use of P-glycoprotein inhibitors Pharm. Res. 21 261–270 Occurrence Handle15032307 Occurrence Handle1:CAS:528:DC%2BD2cXhtlWqt70%3D Occurrence Handle10.1023/B:PHAM.0000016238.44452.f1
B. Heurtault, P. Saulnier, J. P. Benoit, J. E. Proust, B. Pech, and J. Richard. Nanocapsules comprising (semi-) liquid lipid core and solid lipid shell, prepared by phase inversion methods, useful for administration of lipophilic or hydrophilic drugs by injection, orally or intranasally, 2000. Patent WO 01/64328.
B. Heurtault P. Saulnier B. Pech J. E. Proust J. P. Benoit (2002) ArticleTitleA novel phase inversion-based process for the preparation of lipid nanocarriers Pharm. Res. 19 875–880 Occurrence Handle12134960 Occurrence Handle1:CAS:528:DC%2BD38XltVSgtrs%3D Occurrence Handle10.1023/A:1016121319668
J. S. Coon W. Knudson K. Clodfelter B. Lu R. S. Weinstein (1991) ArticleTitleSolutol HS 15, nontoxic polyoxyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance Cancer Res. 51 897–902 Occurrence Handle1988130 Occurrence Handle1:CAS:528:DyaK3MXhtlGltbw%3D
J. P. Benoit and A. Lamprecht. Use of P-glycoprotein inhibitor surfactants at the surface of a colloidal carrier, 2004. Patent WO2004071498.
K. H. Diehl R. Hull D. Morton R. Pfister Y. Rabemampianina D. Smith J. M. Vidal C. Vorstenbosch Particlevan de (2001) ArticleTitleA good practice guide to the administration of substances and removal of blood, including routes and volumes J. Appl. Toxicol. 21 15–23 Occurrence Handle11180276 Occurrence Handle1:CAS:528:DC%2BD3MXht1Kmurw%3D Occurrence Handle10.1002/jat.727
A. Lamprecht Y. Bouligand J. P. Benoit (2002) ArticleTitleNew lipid nanocapsules exhibit sustained release properties for amiodarone J. Control. Release 84 59–68 Occurrence Handle12399168 Occurrence Handle1:CAS:528:DC%2BD38XnvFOnsrY%3D Occurrence Handle10.1016/S0168-3659(02)00258-4
M. Pereira de Oliveira, E. Garcion, N. Venisse, J. P. Benoit, W. Couet, and J. C. Olivier. Tissue distribution of indinavir administered as solid lipid nanocapsule formulation in mdr1a (+/+) and mdr1a (−/−) CF-1 mice. Pharm. Res. 22:1898–1905 (2005).
C. Dulieu D. Bazile (2005) ArticleTitleInfluence of lipid nanocapsules composition on their aptness to freeze-drying Pharm. Res. 22 285–292 Occurrence Handle15783077 Occurrence Handle1:CAS:528:DC%2BD2MXitlClsbc%3D Occurrence Handle10.1007/s11095-004-1196-0
J. S. Choi B. W. Jo Y. C. Kim (2004) ArticleTitleEnhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin Eur. J. Pharm. Biopharm. 57 313–318 Occurrence Handle15018990 Occurrence Handle1:CAS:528:DC%2BD2cXhslWlurY%3D Occurrence Handle10.1016/j.ejpb.2003.11.002
J. D. Adams, K. P. Flora, B. R. Goldspiel, J. W. Wilson, S. G. Arbuck, and R. Finley. Taxol: a history of pharmaceutical development and current pharmaceutical concerns. J. Natl. Cancer Inst. Monogr. 15:141–147 (1993).
K. S. Lown R. R. Mayo A. B. Leichtman H. L. Hsiao D. K. Turgeon P. Schmiedlin-Ren M. B. Brown W. Guo S. J. Rossi L. Z. Benet P. B. Watkins (1997) ArticleTitleRole of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine Clin. Pharmacol. Ther. 62 248–260 Occurrence Handle9333100 Occurrence Handle1:CAS:528:DyaK2sXmslOrt7g%3D Occurrence Handle10.1016/S0009-9236(97)90027-8
B. Monsarrat, P. Alvinerie, M. Wright, J. Dubois, F. Gueritte-Voegelein, D. Guenard, R. C. Donehower, and E. K. Rowinsky. Hepatic metabolism and biliary excretion of Taxol in rats and humans. J. Natl. Cancer Inst. Monogr. 15:39–46 (1993).
J. M. Meerum Terwogt M. M. Malingre J. H. Beijnen W. W. Bokkel Huinink Particleten H. Rosing F. J. Koopman O. Tellingen Particlevan M. Swart J. H. Schellens (1999) ArticleTitleCoadministration of oral cyclosporin A enables oral therapy with paclitaxel Clin. Cancer Res. 5 3379–3384 Occurrence Handle10589748 Occurrence Handle1:STN:280:DC%2BD3c%2FlsFKltw%3D%3D
L. E. Buckingham M. Balasubramanian R. M. Emanuele K. E. Clodfelter J. S. Coon (1995) ArticleTitleComparison of solutol HS 15, Cremophor EL and novel ethoxylated fatty acid surfactants as multidrug resistance modification agents Int. J. Cancer 62 436–442 Occurrence Handle7635569 Occurrence Handle1:CAS:528:DyaK2MXpsVWms7o%3D
W. N. Charman (2000) ArticleTitleLipids, lipophilic drugs, and oral drug delivery—some emerging concepts J. Pharm. Sci. 89 967–978 Occurrence Handle10906720 Occurrence Handle1:CAS:528:DC%2BD3cXmtVWms7c%3D Occurrence Handle10.1002/1520-6017(200008)89:8<967::AID-JPS1>3.0.CO;2-R
S. M. Caliph W. N. Charman C. J. Porter (2000) ArticleTitleEffect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats J. Pharm. Sci. 89 1073–1084 Occurrence Handle10906731 Occurrence Handle1:CAS:528:DC%2BD3cXmtVWmsb4%3D Occurrence Handle10.1002/1520-6017(200008)89:8<1073::AID-JPS12>3.0.CO;2-V
Y. Jiao N. Ubrich M. Marchand-Arvier C. Vigneron M. Hoffman T. Lecompte P. Maincent (2002) ArticleTitleIn vitro and in vivo evaluation of oral heparin-loaded polymeric nanoparticles in rabbits Circulation 105 230–235 Occurrence Handle11790706 Occurrence Handle1:CAS:528:DC%2BD38XhsFehtbs%3D Occurrence Handle10.1161/hc0202.101988
M. Aprahamian C. Michel W. Humbert J. P. Devissaguet C. Damge (1987) ArticleTitleTransmucosal passage of polyalkylcyanoacrylate nanocapsules as a new drug carrier in the small intestine Biol. Cell 61 69–76 Occurrence Handle2833966 Occurrence Handle1:CAS:528:DyaL1cXhsFKgtL0%3D
M. M. Malingre J. H. Beijnen H. Rosing F. J. Koopman O. Tellingen Particlevan K. Duchin W. W. Bokkel Huinink ParticleTen M. Swart J. Lieverst J. H. Schellens (2001) ArticleTitleA phase I and pharmacokinetic study of bi-daily dosing of oral paclitaxel in combination with cyclosporin A Cancer Chemother. Pharmacol. 47 347–354 Occurrence Handle11345652 Occurrence Handle1:CAS:528:DC%2BD3MXjt1Chsbc%3D Occurrence Handle10.1007/s002800000226
Acknowledgments
We are very grateful to Pierre Legras and Jerome Roux from the animal house of the Medical College of Angers for skilful technical support. We would also like to thank Drs. Claire Dulieu and Didier Bazile from Mainelab/Ethypharm for supplying the batches of lipid nanocapsules. This work was supported by grants from the “Fondation pour la Recherche Médicale”, the “Comité départemental du Maine-et-Loire de la Ligue contre le Cancer”, and the “Association pour la Recherche sur le Cancer”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peltier, S., Oger, JM., Lagarce, F. et al. Enhanced Oral Paclitaxel Bioavailability After Administration of Paclitaxel-Loaded Lipid Nanocapsules. Pharm Res 23, 1243–1250 (2006). https://doi.org/10.1007/s11095-006-0022-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-0022-2