Abstract
Objectives
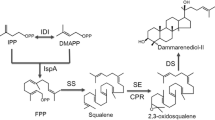
To genetically engineer Escherichia coli for the heterologous biosynthesis of triterpenoid, ambrein, the main bioactive component of ambergris, by constituting a novel squalene-derived ambrein biosynthetic pathway in E. coli.
Results
The ScERG9 gene encoding the squalene synthase (SS) was integrated into the E. coli genome to generate a squalene-producing strain that supplied the central precursor squalene for the formation of cyclic triterpenoids. The mutated squalene–hopene synthase (D377C SHC) and the tetraprenyl-β-curcumene cyclase (BmeTC) were co-expressed with SS to construct a novel ambrein biosynthetic pathway in E. coli. Ambrein was produced at 2.6 mg l−1.
Conclusions
An E. coli chassis for ambrein production was constructed by combining the squalene synthesis module with the downstream cyclization module.



Similar content being viewed by others
References
Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330:70–74
Jing Z, Li Q, Tao S, Zhu X, Xu H, Tang J, Zhang X, Ma Y (2013) Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab Eng 17:42–50
Katabami A, Li L, Iwasaki M, Furubayashi M, Saito K, Umeno D (2015) Production of squalene by squalene synthases and their truncated mutants in Escherichia coli. J Biosci Bioeng 119:165–171
Kovatcheva A, Golbraikh A, Oloff S, Xiao YD, Zheng W, Wolschann P, Buchbauer G, Tropsha A (2004) Combinatorial QSAR of ambergris fragrance compounds. J Chem Inf Comput Sci 44:582–595
Li Y, Lin Z, Huang C, Zhang Y, Wang Z, Tang Y, Chen T, Zhao X (2015) Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab Eng 31:13–21
Li D, Zhang Q, Zhou Z, Zhao F, Lu W (2016) Heterologous biosynthesis of triterpenoid dammarenediol-II in engineered Escherichia coli. Biotechnol Lett 38:603–609
Ohloff G, Schulte-Elte KH, Müller BL (1977) Formation of ambergris odorants from ambrein under simulated natural conditions. Helv Chim Acta 60:2763–2766
Sato T, Hoshino T (1999) Functional analysis of the DXDDTA motif in squalene–hopene cyclase by site-directed mutagenesis experiments: initiation site of the polycyclization reaction and stabilization site of the carbocation intermediate of the initially cyclized A-ring. Biosci Biotechnol Biochem 63:2189–2198
Sato T, Hoshino H, Yoshida S, Nakajima M, Hoshino T (2011) Bifunctional triterpene/sesquarterpene cyclase: tetraprenyl-β-curcumene cyclase is also squalene cyclase in Bacillus megaterium. J Am Chem Soc 133:17540–17543
Shen YC, Cheng SY, Kuo YH, Hwang TL, Chiang MY, Khalil AT (2007) Chemical transformation and biological activities of ambrein, a major product of ambergris from Physeter macrocephalus (sperm whale). J Nat Prod 70:147–153
Taha SA (1989) Chemical investigation of the internal secretion of the sperm blue whale. Pak J Pharm Sci 2:105–110
Taha SA (1992) Studies on the mode of action of ambrein as a new antinociceptive compound. Jpn J Pharmacol 60:67–71
Taha SA, Islam MW, Ageel AM (1995) Effect of ambrein, a major constituent of ambergris, on masculine sexual behavior in rats. Arch Int Pharmacodyn Ther 329:283–294
Taha SA, Raza M, El-Khawad IE (1998) Effect of ambrein on smooth muscle responses to various agonists. J Ethnopharmacol 60:19–26
Tanimoto H, Oritani T (1997) Synthesis of (+)-ambrein. Tetrahedron 53:3527–3536
Ueda D, Hoshino T, Sato T (2013) Cyclization of squalene from both termini: identification of an onoceroid synthase and enzymatic synthesis of ambrein. J Am Chem Soc 135:18335–18338
Zhang H, Qiang L, Cao Y, Feng X, Zheng Y, Zou H, Hui L, Yang J, Mo X (2014) Microbial production of sabinene—a new terpene-based precursor of advanced biofuel. Microb Cell Factories 13:20
Acknowledgements
The present work was funded by the National Basic Research Program of China (“973” Program: 2012CB721105) and the Major Research Plan of Tianjin (16YFXTSF00460).
Supporting information
Supplementary method—effective prediction of relative response factor RRF for epifriedelanol/ambrein in GC-FID analysis via a carbon number-based approach.
Supplementary Table 1—strains and plasmids used.
Supplementary Table 2—DNA sequences of exogenous genes used for the construction of the ambrein biosynthetic pathway in Escherichia coli.
Supplementary Table 3—oligonucleotide primers used in this study.
Supplementary Fig. 1—identification of the authentic ambergris containing ambrein via GC–MS.
Supplementary Fig. 2—quantitative analysis of ambrein production via GC-FID.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ke, D., Caiyin, Q., Zhao, F. et al. Heterologous biosynthesis of triterpenoid ambrein in engineered Escherichia coli . Biotechnol Lett 40, 399–404 (2018). https://doi.org/10.1007/s10529-017-2483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2483-2