Abstract
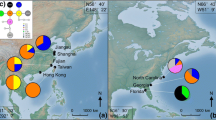
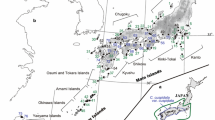
The wild flowering cherry Prunus lannesiana var. speciosa is highly geographically restricted, being confined to the Izu Islands and neighboring peninsulas in Japan. In an attempt to elucidate how populations of this species have established we investigated the genetic diversity and differentiation in seven populations (sampling 408 individuals in total), using three kinds of genetic markers: chloroplast DNA (cpDNA), amplified fragment length polymorphisms (AFLPs), and 11 nuclear SSR polymorphic loci. Eight haplotypes were identified based on the cpDNA sequence variations, 64 polymorphic fragments were scored for the AFLP markers, and a total of 154 alleles were detected at the 11 nuclear SSR loci. Analysis of molecular variance showed that among-population variation accounted for 16.55, 15.04 and 7.45% of the total detected variation at the cpDNA, AFLPs, and SSR loci, respectively. Thus, variation within populations accounted for most of the genetic variance for all types of markers, although the genetic differentiation among populations was also highly significant. For cpDNA variation, no clear structure was found among the populations, except that of the most distant island, although an “isolation by distance” pattern was found for each marker. Both neighbor-joining trees and structure analysis indicate that the genetic relationships between populations reflect geological variations between the peninsula and the islands and among the islands. Furthermore, hybridization with related species may have affected the genetic structure, and some genetic introgression is likely to have occurred.




Similar content being viewed by others
References
Arnold ML, Emms SK (1998) Molecular markers, gene flow, and natural selection. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II. DNA sequencing. Kluwer, Dordrecht, pp 442–458
Barrett SCH (1998) The reproductive biology and genetics of island plants. In: Grand PR (ed) Evolution on islands. Oxford University Press, Oxford, pp 18–34
Barton NH (1998) Natural selection and random genetic drift as causes of evolution on islands. In: Grand PR (ed) Evolution on islands. Oxford University Press, Oxford, pp 102–123
Brunsfeld SJ, Soltis DE, Soltis PS (1992) Evolutionary pattern and processes in Salix sect. Logifoliae: evidence from chloroplast DNA. Syst Bot 17:239–256
Cain SA (1944) Foundations of plant geography. Harper & Sons, New York
Carlquist S (1974) Island biology. Columbia Univ Press, Columbia, 660 pp
Cipriani G, Lot G, Huang WG, Marrazzo MT, Peterlunger E, Testolin R (1999) AC/GT and AG/CT microsatellite repeats in peach [Prunus persica (L.) Batsch]: isolation, characterisation and cross-species amplification in Prunus. Theor Appl Genet 99:65–72
Coart E, Vekemans X, Smulders MJM, Wagner I, Van Huylenbroeck J, Van Bockstaele E, Roldán-Ruiz I (2003) Genetic variation in the endangered wild apple (Malus sylvestris (L.) Mill.) in Belgium as revealed by AFLP and microsatellite markers. Consequences for conservation. Mol Ecol 12:845–857
Demesure B, Sodzi N, Petit RJ (1995) A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Mol Ecol 4:129–131
Dieringer D, Schlotterer C (2003) MICROSATELLITE ANALYSER (MSA): a platform independent analysis tool for large microsatellite data sets. Mol Ecol Notes 3:167–169
Dirlewanger E, Cosson P, Tavaud M, Aranzana MJ, Poizat C, Zanetto A, Arús P, Laigret F (2002) Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor Appl Genet 105:127–138
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Ennos RA (1994) Estimating the relative rates of pollen and seed migration among plant populations. Heredity 72:250–259
Estoup A, Jarne P, Cornuet JM (2002) Homoplasy and mutation model at microsatellite loci and their consequences for population genetics analysis. Mol Ecol 11:1591–1604
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Felsenstein J (1989) PHYLIP––phylogeny inference package (Version 32). Cladistics 5:164–166
Fineschi S, Salvini D, Turchini D, Pastorelli R, Vendramin GG (2005) Crataegus monogyna Jacq. and C. laevigata (Poir.) DC. (Rosaceae, Maloideae) display low level of genetic diversity assessed by chloroplast markers. Plant Syst Evol 250:187–196
Goldstein DB, Schlötterer C (1999) Microsatellites. Evolution and application. Oxford University Press, New York
Gornitz V (1995) Monitoring sea level changes. Clim Chang 31:515–544
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm
Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with interspecific variation. Mol Ecol 8:521–523
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. New Forests 6:95–124
Hedrick PW (1999) Perspective: highly variable loci and their interpretation in evolution and conservation. Evolution 53:313–318
Iwata H, Kamijo T, Tsumura Y (2006) Assessment of genetic diversity of native species in Izu Islands for a discriminate choice of source populations: implications for revegetation of volcanically devastated sites. Conserv Genet 7:399–413
Japan Meteorological Agency (2005) National catalogue of the active volcanoes in Japan, 3rd edn. National Printing Bureau (in Japanese)
Kalinowski ST (2004) Counting alleles with rarefaction: private alleles and hierarchical sampling designs. Conserv Genet 5:539–543
Kalinowski ST (2005) HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol 5:187–189
Lewontin RC (1972) The apportionment of human diversity. Evol Biol 6:381–398
Maguire TL, Peakall R, Saenger P (2002) Comparative analysis of genetic diversity in the mangrove species Avicennia marina (Forsk.) Vierh. (Avicenniaceae) detected by AFLPs and SSRs. Theor Appl Genet 104:388–398
Makino T (1961) Makino’s new illustrated flora of Japan. Hokuryukan Publishing, Tokyo (in Japanese)
Mantel NA (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mariette S, Cottrell J, Csaikl UM, Goikoechea P, König A, Lowe AJ, Van Dam BC, Barreneche T, Bodénès C, Streiff R, Burg K, Groppe K, Munro RC, Tabbener H, Kremer A (2002a) Comparison of levels of genetic diversity detected with AFLP and microsatellite markers within and among mixed Q. petraea (MATT.) LIEBL. and Q. robur L. stands. Silvae Genet 51:72–79
Mariette S, Le Corre V, Austerlitz F, Kremer A (2002b) Sampling within the genome for measuring within population diversity: trade-offs between markers. Mol Ecol 11:1145–1156
McCauley DE (1995) The use of chloroplast DNA polymorphisms in studies of gene flow in plants. Trends Ecol Evol 10:198–202
Mohanty A, Martín JP, Aguinagalde I (2000) Chloroplast DNA diversity within and among populations of the allotetraploid Prunus spinosa L. Theor Appl Genet 100:1304–1310
Mohanty A, Martín JP, Aguinagalde I (2001) A population genetic analysis of chloroplast DNA in wild populations of Prunus avium L. in Europe. Heredity 87:421–427
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Nat Acad Sci USA 70:3321–3323
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nei M, Takezaki N (1994) Estimation of genetic distances and phylogenetic trees from DNA analysis. In: Smith C (ed) Proceedings of the 5th World Congress of Genetics Applied to Livestock Production. University of Guelph, Canada, pp 405–412
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenic trees from molecular data. J Mol Evol 19:153–170
Newton AC, Allnutt TR, Gillies ACM, Lowe AJ, Ennos RA (1999) Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends Ecol Evol 14:140–145
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Ohba H, Kawasaki T, Tanaka H (2007) Flowering cherries of Japan, New Edition. Yama-to-Keikoku-Sha, Tokyo (in Japanese)
Ono M, Sugawara T (1981) An analysis of the flowering plant flora of the Ogasawara (Bonin) Islands with regard to their mode of dispersal. Ogasawara Res 4 & 5:25–40 (in Japanese)
Page RDM (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Comp Appl Biosci 12:357–358
Petit RJ, Aguinagalde I, de Beaulieu JL, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohanty A, Müller-Starck G, Demesure-Musch B, Palmé A, Martín JP, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565
Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90:502–503
Powell W, Morgante M, Andre C, Hanafey M, Vogel J, Tingey S, Rafalski A (1996) The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pritchard JK, Wen W, Falush D (2007) Documentation for STRUCTURE software: version 2.2. University of Chicago, Chicago, USA. http://pritch.bsd.uchicago.edu/
Raymond M, Rousset F (1995) GENEPOP: population genetics software for exact test and ecumenicism. J Hered 86:248–249
Rieseberg LH, Soltis DE (1991) Phylogenetic consequences of cytoplasmic gene flow in plants. Evol Trends Plants 5:65–84
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schneider S, Roessli D, Excoffier L (2000) Arlequin, Version 2.000: a software for population genetics data analysis. Genetics and Biometry Laboratory, University of Geneva, Geneva
Smulders MJM, van der Schoot J, Geerts RHEM, Antonisse-de Jong AG, Korevaar H, van der Werf A, Vosman B (2000) Genetic diversity and the reintroduction of meadow species. Plant Biol 2:447–454
Sosinski B, Gannavarapu M, Hager LD, Beck LE, King GJ, Ryder CD, Rajapakse S, Baird WV, Ballard RE, Abbott AG (2000) Characterization of microsatellite markers in peach [Prunus persica (L.) Batsch]. Theor Appl Genet 101:421–428
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Takahashi H (1971) Fossa Magna element plants. Res Report Kanagawa Prefect Museum. Nat History 2:1–63
Terachi T (1993) Structural alterations of chloroplast genome and their significance to the higher plant evolution. Bull Inst Natl Land Util Dev Kyoto Sangyo Univ 14:138–148
Testolin R, Marrazzo T, Cipriani G, Quarta R, Verde I, Dettori MT, Pancaldi M, Sansavini S (2000) Microsatellite DNA in peach (Prunus percica L. Batsch) and its use fingerprinting and testing the genetic origin of cultivars. Genome 43:512–520
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsuda Y, Kimura M, Kato S, Katsuki T, Mukai Y, Tsumura Y (2009) Genetic structure of Cerasus jamasakura, a Japanese flowering cherry, revealed by nuclear SSRs: implications for conservation. J Plant Res 122:367–375
van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vos P, Hoger R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Weising K, Gardner R (1999) A set of conserved PCR primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome 42:9–19
Whittemore AT, Schaal BA (1991) Interspecific gene flow in oaks. Proc Natl Acad Sci USA 88:2540–2544
Wolfe AD, Liston A (1998) Contribution of PCR-based methods to plant systematics and evolutionary biology. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II. DNA sequencing. Kluwer, Dordrecht, pp 43–86
Yeh FC, Boyle TJB (1997) Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belgian J Bot 129:157
Acknowledgments
We thank Michihal and Junko Kato, Yoshinari Moriguchi and Tomokazu Takahashi for their practical help with the sample collection, and Jungo Yuzurihara and Yasuomi Ota for their help with preserving the samples. This study was partly supported within the research budget of the Forest Recovery Project for the Disaster Area on Miyake Island, provided by the Tokyo Metropolitan Government, as well as a grant for Research on genetic guidelines based on molecular population analysis of plants for restoration projects from the Ministry of Environment, Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kato, S., Iwata, H., Tsumura, Y. et al. Genetic structure of island populations of Prunus lannesiana var. speciosa revealed by chloroplast DNA, AFLP and nuclear SSR loci analyses. J Plant Res 124, 11–23 (2011). https://doi.org/10.1007/s10265-010-0352-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-010-0352-3