Abstract
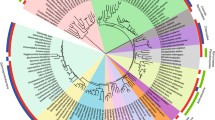
Stability and function of a large number of proteins are crucially dependent on the presence of disulfide bonds. Recent genome analysis has pointed out an important role of disulfide bonds for the structural stabilization of intracellular proteins from hyperthermophilic archaea and bacteria. These findings contradict the conventional view that disulfide bonds are rare in those proteins. A specific protein, known as protein disulfide oxidoreductase (PDO) is recognized as a potential key enzyme in intracellular disulfide-shuffling in hyperthermophiles. The structure of this protein consists of two combined thioredoxin-related units which together, in tandem-like manner, form a closed protein domain. Each of these units contains a distinct CXXC active site motif. Both sites seem to have different redox properties. A relation to eukaryotic protein disulfide isomerase is suggested by the observed structural and functional characteristics of the protein. Enzymological studies have revealed that both, the archaeal and bacterial forms of this protein show oxidative and reductive activity and are able to isomerize protein disulfides. The variety of active site disulfides found in PDO’s from hyperthermophiles is puzzling. It is assumed, that PDO enzymes in hyperthermophilic archaea and bacteria may be part of a complex system involved in the maintenance of protein disulfide bonds.







Similar content being viewed by others
Abbreviations
- PDI:
-
Protein disulfide isomerase
- PDO:
-
Protein disulfide oxido-reductase
- DTT:
-
Dithiothreitol
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
References
Anfinsen CB, Haber E (1961) studies on the reduction and re-formation of protein disulfide bonds. J Biol Chem 236:1361–1363
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181:223–230
Aslund F, Berndt KD, Holmgren A (1997) Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein-protein redox equilibria. J Biol Chem 272:30780–86
Bardwell JCA, Beckwith J (1993) The bonds that tie: catalyzed disulfide bond formation. Cell 74:769–711
Bardwell JC, McGovern K, Beckwith J (1991) Identification of a protein required for disulfide bond formation in vivo. Cell 67:581–589
Beeby M, O’Connor BD, Ryttersgaard C, Boutz DR, Perry LJ, Yeates TO (2005) The genomics of disulfide bonding and protein stabilization in thermophiles. PloS Biol 3:1–10
Bönisch H, Schmidt CL, Schäfer G, Ladenstein R (2002) The structure of the soluble domain of an archaeal rieske iron–sulfur protein at 1.1 Å resolution. J Mol Biol 319:791–805
Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C (2002) Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295:1073–1077
D’Ambrosio K, DeSimone G, Pedone E, Rossi M, Bartolucci S, Pedone C (2004) Crystallization and preliminary X-ray diffraction studies of a protein disulfide oxidoreductase from Aquifex aeolicus. Acta Cryst D 60:2076–2077
Darby NJ, Freedman RB, Creighton TE (1994) Dissecting the mechanism of protein disulfide isomerase: catalysis of disulfide bond formation in a model peptide. Biochemistry 33:7937–7947
Darby NJ, Creighton TE (1995) Functional properties of the individual thioredoxin-like domains of protein disulfide isomerase. Biochemistry 34:11725–11735
Darby NJ, Kemmink J, Creighton TE (1996) Identifying and characterizing a structural domain of protein disulfide isomerase. Biochemistry 35:10517–10528
Derman AI, Beckwith J (1991) Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J Bacteriol 173:7719–7722
Derman AI, Prinz WA, Belin D, Beckwith J (1993) Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science 262:1744–1747
Eklund H, Ingelman M, Söderberg BO, Uhlin T, Nordlund P, Nikkola M, Sonnerstam U, Joelson T, Petratos K (1992) Structure of oxidized bacteriophage T4 glutaredoxin (thioredoxin): refinement of native and mutant proteins. J Mol Biol 228:596–618
Ellgaard L, Ruddock LW (2005) The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep 6:28–32
Fomenko DE, Gladyshev VN (2003) Identity and functions of CXXC-derived motifs. Biochemistry 42:1214–11225
Freedman RB (1995) The formation of protein disulfide bonds. Curr Opin Struct Biol 5:85–91
Freedman RB, Hirst TR, Tuite MF (1994) Protein disulfide isomerase: building bridges in protein folding. Trends Biochem Sci 19:331–336
Guagliardi A, de Pascale D, Cannio R, Nobile V, Bartolucci S, Rossi M (1995) The purification, cloning, and high level expression of a glutaredoxin-like protein from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 270, 5748–5755, with a published erratum in J Biol Chem (1997) 272, 20961
Holmgren A (1972) Tryptophan fluorescence study of conformational transitions of the oxidized and reduced form of thioredoxin. J Biol Chem 247:1992–1998
Holmgren A (1979) Thioredoxin catalyzes the reduction of insuline disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem 254:9627–9632
Holmgren A (1995) Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 3:239–243
Holmgren A, Söderberg BO, Eklund H, Brändén CI (1975) Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 Å resolution. Proc Natl Acad Sci USA 72:2305–2309
Huber-Wunderlich M, Glockshuber R (1998) A single dipeptide sequence determines the redox properties of a whole enzyme family. Fold Des 3:161–171
Hutchinson EG, Thornton JM (1996) PROMOTIF-A program to identify and analyze structural motifs in proteins. Protein Sci 5:212–220
Kadokura H, Katzen F, Beckwith J (2003) Protein disulfide bond formation in prokaryotes. Annu Rev Biochem 72:111–135
Karlström M, Stokke R, Steen IH, Birkeland NK, Ladenstein R (2005) Isocitrate dehydrogenase from the hyperthermophile Aeropyrum pernix: X-ray structure analysis of a ternary enzyme-substrate complex and thermal stability. J Mol Biol 345:559–577
Karshikoff A, Ladenstein R (2001) Ion pairs and the thermotolerance of proteins from hyperthermophiles: a “traffic rule” for hot roads. TiBS 26:550–556
Kashima Y, Ishikawa K (2003) A hyperthermostable novel protein-disulfide oxidoreductase is reduced by thioredoxin reductase from the hyperthermophilic archaeon Pyrococcus horikoshii. Arch Biochem Biophys 418:179–185
Katti SK, LeMaster DM, Eklund H (1990) Crystal structure of thioredoxin from Escherichia coli at 1.68 Å resolution. J Mol Biol 212:167–184
Katz BA, Kossiakoff A (1986) The crystallographically determined structures of atypical strained disulfides engineered into subtilisin. J Biol Chem 261:15480–15485
Kemmink J, Darby N, Dijkstra K, Nilges M, Creighton TE (1996) Structure determination of the N-terminal thioredoxin-like domain of protein disulfide isomerase using multidimensional heteronuclear 13C/15N NMR spectroscopy. Biochemistry 35:7684–7691
Krause G, Lundström J, Barea JL, Delacues CP, Holmgren A (1991) Mimicking the active site of protein disulfide isomerase by substitution of proline 34 in Escherichia coli thioredoxin. J Biol Chem 266:9494–9500
Kulp MH, Frickel EM, Ellgaard L, Weissman JS (2006) Domain architecture of protein-disulfide isomerase facilitates its dual role as an oxidase and an isomerase in ero1p-mediated disulfide formation. J Biol Chem 281:876–884
Lambert N, Freedman RB (1983) Kinetics and specificity of homogeneous protein disulphide-isomerase in protein disulphide isomerization and in thiol–protein–disulphide oxidoreduction. Biochem J 213:235–243
Mallick P, Boutz DR, Eisenberg D, Yeates TO (2002) Genomic evidence that the intercellular proteins of archaeal microbes contain disulfide bonds. Proc Natl Acad Sci USA 99:9679–9684
Martin JL, Bardwell JCA, Kuriyan J (1993) Crystal structure of the DsbA protein required for disulfide bond formation in vivo. Nature 365:464–468
Mössner E, Huber-Wunderlich M, Glockshuber R (1998) Characterization of E. coli thioredoxin variants mimicking the active sites of other thiol-disulfide oxidoreductases. Protein Science 7:1233–1244
Pedone E, Ren B, Ladenstein R, Rossi M, Bartolucci S (2004) Functional properties of the protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus: a member of a novel protein family related to protein disulfide-isomerase. Eur J Biochem 271:3437–3448
Pedone E, D’Ambrosio K, De Simone G, Rossi M, Pedone C, Bartolucci S (2006a) Insights on a new PDI-like family: structural and functional analysis of a protein disulfide oxidoreductase from the bacterium Aquifex aeolicus. J Mol Biol 356:155–164
Pedone E, Limauro D, D’Alterio R, Rossi M, Bartolucci S (2006b) Characterization of a multifunctional protein disulfide oxidoreductase from Sulfolobus solfataricus. FEBS J 273:5407–5420
Pigiet VP, Schuster BJ (1986) Thioredoxin-catalyzed refolding of disulfide-containing proteins. Proc Natl Acad Sci USA 83:7643–7647
Prinz WA, Aslund F, Holmgren A, Beckwith J (1997) The role of the thioredoxin and glutaredoxin pathways in reducing disulfide bonds in the Escherichia coli cytoplasm. J Biol Chem 272:15661–15667
Ren B, Tibbelin G, de Pascale D, Rossi M, Bartolucci S, Ladenstein R (1998) A protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus contains two thioredoxin fold units. Nat Struct Biol 5:602–611
Ren B, Haase I, Fischer M, Ladenstein R (2006) unpublished work
Ruddock LW, Hirst TR, Freedman RB (1996) pH dependence of the dithiol-oxidizing activity of DsbA (a periplasmic protein thiol: disulphide oxidoreductase) and protein disulphide isomerase: studies with a novel simple peptide substrate. Biochem J 315:1000–1005
Tian G, Xiang S, Noiva R, Lennarz WJ, Schindelin H (2006) The crystal structure of yeast protein disulfide isomerase suggests cooperativity between its active sites. Cell 124:61–73
Toth EA, Worby C, Dixon JE, Goedken ER, Marqusee S, Yeates TO (2000) The crystal structure of adenylosuccinate lyase from Pyrobaculum aerophilum reveals an intracellular protein with three disulfide bonds. J Mol Biol 301:433–450
Vuori K, Myllylä R, Pihlajaniemi T, Kivirikko KI (1992) Expression and site-directed mutagenesis of human protein disulfide isomerase in Escherichia coli. This multifunctional polypeptide has two independently acting catalytic sites for the isomerase activity. J Biol Chem 267:7211–7214
Wang Q, Chang A (1999) Eps1, a novel PDI-related protein involved in ER quality control in yeast. EMBO J 18:5972–5982
Wunderlich M, Jaenicke R, Glockshuber R (1993) The redox properties of protein disulfide isomerase (DsbA) of Escherichia coli result from a tense conformation of its oxidized form. J Mol Biol 233:559–566
Zapun A, Bardwell JC, Creighton TE (1993) The reactive and destabilizing disulfide bond of DsbA, a protein required for disulfide bond formation in vivo. Biochemistry 32:5083–5092
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D.A. Cowan.
Rights and permissions
About this article
Cite this article
Ladenstein, R., Ren, B. Reconsideration of an early dogma, saying “there is no evidence for disulfide bonds in proteins from archaea”. Extremophiles 12, 29–38 (2008). https://doi.org/10.1007/s00792-007-0076-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0076-z