Abstract
Background
Glioma invading the corpus callosum (CC) accounts for approximately 14% of gliomas and is thought to be more aggressive. However, there is still a lack of studies on the pathogenesis and molecular features of this condition. Here, we examined the occurrence association of CC invasion with respect to patients’ clinical, pathological, and genetic characteristics.
Methods
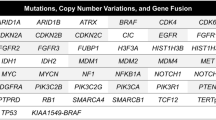
First, a cohort of 331 patients was included, with 86 cases (26%) that were diagnosed with invasion glioma. They were all analyzed for basic clinical and pathological characteristics and four routinely tested glioma molecular markers. Second, 29 pairs of patients who underwent deep sequencing of 68 glioma molecular alterations were selected from both groups for in-depth analysis.
Results
The results of the first part showed that there was no difference between the two groups in terms of the basic factors in univariate analysis, while in multivariate logistic analysis, WHO grade was the risk factor for CC invasion (p = 0.001). The results of the second part showed that the paired groups had different genetic expression profiles, which highlighted glioma invading the CC as a distinct biological entity. PDGFRA mutation (PDGFRAmut) was present in 9 patients with invasive gliomas (31%), but only in one case (3.4%) in the control group (OR 17.331; 95% CI 1.987–151.156).
Conclusion
Our data revealed the clinical, pathological, and genetic characteristics of glioma invading the CC and showed that it may be associated with glioma WHO grade and PDGFRAmut, but not other factors. Thus, the risk signaling pathway may offer potential therapeutic targets for this disease.



Similar content being viewed by others
References
Agrawal A (2009) Butterfly glioma of the corpus callosum. J Cancer Res Ther 5:43–45
Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411:355–365. https://doi.org/10.1038/35077225
Bourekas EC, Varakis K, Bruns D, Christoforidis GA, Baujan M, Slone HW, Kehagias D (2002) Lesions of the corpus callosum: MR imaging and differential considerations in adults and children. AJR Am J Roentgenol 179:251–257. https://doi.org/10.2214/ajr.179.1.1790251
Chaichana KL, Jusue-Torres I, Lemos AM, Gokaslan A, Cabrera-Aldana EE, Ashary A, Olivi A, Quinones-Hinojosa A (2014) The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neuro-Oncol 120:625–634. https://doi.org/10.1007/s11060-014-1597-9
Chen KT, Wu TW, Chuang CC, Hsu YH, Hsu PW, Huang YC, Lin TK, Chang CN, Lee ST, Wu CT, Tseng CK, Wang CC, Pai PC, Wei KC, Chen PY (2015) Corpus callosum involvement and postoperative outcomes of patients with gliomas. J Neuro-Oncol 124:207–214. https://doi.org/10.1007/s11060-015-1823-0
Dayani F, Young JS, Bonte A, Chang EF, Theodosopoulos P, McDermott MW, Berger MS, Aghi MK (2018) Safety and outcomes of resection of butterfly glioblastoma. Neurosurg Focus 44:E4. https://doi.org/10.3171/2018.3.FOCUS1857
Dziurzynski K, Blas-Boria D, Suki D, Cahill DP, Prabhu SS, Puduvalli V, Levine N (2012) Butterfly glioblastomas: a retrospective review and qualitative assessment of outcomes. J Neuro-Oncol 109:555–563. https://doi.org/10.1007/s11060-012-0926-0
Lapin DH, Tsoli M, Ziegler DS (2017) Genomic insights into diffuse intrinsic Pontine Glioma. Front Oncol 7:57. https://doi.org/10.3389/fonc.2017.00057
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Monaco EA 3rd, Armah HB, Nikiforova MN, Hamilton RL, Engh JA (2011) Grade II oligodendroglioma localized to the corpus callosum. Brain Tumor Pathol 28:305–309. https://doi.org/10.1007/s10014-011-0054-0
Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS, Onar-Thomas A, Baker JN, Gajjar A, Ellison DW, Baker SJ (2011) Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 29:3999–4006. https://doi.org/10.1200/JCO.2011.35.5677
Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG (2010) Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-Oncology 12:520–527. https://doi.org/10.1093/neuonc/nop066
Rees JH, Smirniotopoulos JG, Jones RV, Wong K (1996) From the archives of the AFIP - Glioblastoma multiforme: radiologic-pathologic correlation. Radiographics 16:1413–1438. https://doi.org/10.1148/radiographics.16.6.8946545
Renard D, Castelnovo G, Campello C, Bouly S, Le Floch A, Thouvenot E, Waconge A, Taieb G (2014) An MRI review of acquired corpus callosum lesions. J Neurol Neurosurg Psychiatry 85:1041–1048. https://doi.org/10.1136/jnnp-2013-307072
Reuss DE, Sahm F, Schrimpf D, Wiestler B, Capper D, Koelsche C, Schweizer L, Korshunov A, Jones DT, Hovestadt V, Mittelbronn M, Schittenhelm J, Herold-Mende C, Unterberg A, Platten M, Weller M, Wick W, Pfister SM, von Deimling A (2015) ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an "integrated" diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol 129:133–146. https://doi.org/10.1007/s00401-014-1370-3
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2:494–503; quiz 1 p following 516. https://doi.org/10.1038/ncpneuro0289
Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She YH, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439:358–362. https://doi.org/10.1038/nature04304
Takami H, Yoshida A, Fukushima S, Arita H, Matsushita Y, Nakamura T, Ohno M, Miyakita Y, Shibui S, Narita Y, Ichimura K (2015) Revisiting TP53 mutations and immunohistochemistry--a comparative study in 157 diffuse gliomas. Brain Pathol 25:256–265. https://doi.org/10.1111/bpa.12173
Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M, Schulz D, Simon M, Schlegel U, Senft C, Geletneky K, Braun C, Hartung JG, Reuter D, Metz MW, Bach F, Pietsch T (2015) A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer 51:522–532. https://doi.org/10.1016/j.ejca.2014.12.019
Wiestler B, Capper D, Holland-Letz T, Korshunov A, von Deimling A, Pfister SM, Platten M, Weller M, Wick W (2013) ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 126:443–451. https://doi.org/10.1007/s00401-013-1156-z
Yalon M, Rood B, MacDonald TJ, McCowage G, Kane R, Constantini S, Packer RJ (2013) A feasibility and efficacy study of rapamycin and erlotinib for recurrent pediatric low-grade glioma (LGG). Pediatr Blood Cancer 60:71–76. https://doi.org/10.1002/pbc.24142
Acknowledgments
We thank Genetron Health who provided the next-generation sequencing platform, and we appreciate the technique of bioinformatic analysis provided by Liwen Jiang and Lan Su.
Submission declaration and verification
Neither the entire paper nor any part of its content has been published or has been accepted elsewhere. It is not being submitted to any other journal. All authors of this paper have read and approved the final version submitted.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Shaoping Shen and Shiyu Feng are co-first author.
This article is part of the Topical Collection on Tumor – Glioma.
Rights and permissions
About this article
Cite this article
Shen, S., Feng, S., Liu, H. et al. Associations of histological and molecular alterations with invasion of the corpus callosum in gliomas. Acta Neurochir 162, 1691–1699 (2020). https://doi.org/10.1007/s00701-020-04376-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04376-9