Abstract
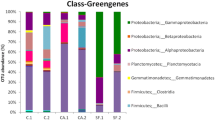
Discoloring biofilms from Cambodian temples Angkor Wat, Preah Khan, and the Bayon and West Prasat in Angkor Thom contained a microbial community dominated by coccoid cyanobacteria. Molecular analysis identified Chroococcidiopsis as major colonizer, but low similarity values (<95%) suggested a similar genus or species not present in the databases. In only two of the six sites sampled were filamentous cyanobacteria, Microcoleus, Leptolyngbya, and Scytonema, found; the first two detected by sequencing of 16S rRNA gene library clones from samples of a moist green biofilm on internal walls in Preah Khan, where Lyngbya (possibly synonymous with Microcoleus) was seen by direct microscopy as major colonizer. Scytonema was detected also by microscopy on an internal wall in the Bayon. This suggests that filamentous cyanobacteria are more prevalent in internal (high moisture) areas. Heterotrophic bacteria were found in all samples. DNA sequencing of bands from DGGE gels identified Proteobacteria (Stenotrophomonas maltophilia and Methylobacterium radiotolerans) and Firmicutes (Bacillus sp., Bacillus niacini, Bacillus sporothermodurans, Lysinibacillus fusiformis, Paenibacillus sp., Paenibacillus panacisoli, and Paenibacillus zanthoxyli). Some of these bacteria produce organic acids, potentially degrading stone. Actinobacteria, mainly streptomycetes, were present in most samples; algae and fungi were rare. A dark-pigmented filamentous fungus was detected in internal and external Preah Khan samples, while the alga Trentepohlia was found only in samples taken from external, pink-stained stone at Preah Khan. Results show that these microbial biofilms are mature communities whose major constituents are resistant to dehydration and high levels of irradiation and can be involved in deterioration of sandstone. Such analyses are important prerequisites to the application of control strategies.





Similar content being viewed by others
References
Adhikary SP, Satapathy DP (1996) Toypothrix byssoidea (Cyanophyceae/Cyanobacteria) from temple rock surfaces of coastal Orissa, India. Nova Hedwigia 62:419–423
Anand N, Mohan E, Hopper RSS, Subramanian TD (1986) Taxonomic studies on blue green algae from certain marine environments. Seaweed Res Util 9:49–56
André MF (2006) Sandstone weathering rates at the Angkor temples (Cambodia). In: Fort R, Alvarez de Buergo M, Gomez-Heras M, Vasquez-Calvo C (eds) Heritage, weathering and conservation. Taylor & Francis, London
André MF, Etienne S, Mercier D, Vautier F, Voldoire O (2008) Assessment of sandstone deterioration at Ta Keo temple (Angkor): first results and future prospects. Environ Geol 56:677–688
Anon (2006) International coordinating committee for the safeguarding and development of the historic Site of Angkor. Report of Fifteenth Technical Committee—June 5, 6, 7, 2006, p 90
Boone DR, Castenholz RW, Garrity GM (2001) Bergey’s manual of systematic bacteriology, vol 1. Springer, New York
Boyer SL, Johansen JR, Flechtner VR, Howard GL (2003) Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S–23S ITS region. J Phycol 38:1222–1235
Crispim CA, Gaylarde CC (2005) Cyanobacteria and biodeterioration of cultural heritage: a review. Microbial Ecol 49:1–9
Crispim CA, Gaylarde CC, Gaylarde PM (2004) Biofilms on church walls in Porto Alegre, RS, Brazil, with special attention to cyanobacteria. Int Biodeter Biodegr 54:121–124
Cullen DW, Hirsch PR (1998) Simple and rapid method for direct extraction of microbial DNA from soil to PCR. Soil Biol Biochem 30:983–993
Darlington A (1981) Ecology of walls. Heinemann, London
Dasman KajiyamaS, Kawasaki H, Yagi M, Seki T, Fukusaki E, Kobayashi A (2002) Paenibacillus glycanilyticus sp. nov., a novel species that degrades heteropolysaccharide produced by the cyanobacterium Nostoc commune. Int J Syst Evol Microbiol 52:1669–1674
Galtier N, Gouy M, Gautier C (1996) SEA VIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci 12:543–548
Gaylarde P, Englert G, Ortega-Morales O, Gaylarde C (2006) Lichen-like colonies of pure Trentepohlia on limestone monuments. Int Biodeter Biodegr 58:119–123
Gaylarde CC, Gaylarde PM (2005) A comparative study of the major microbial biomass of biofilms on exteriors of buildings in Europe and Latin America. Int Biodeter Biodegr 55:131–139
Gaylarde CC, Gaylarde PM, Copp J, Neilan BA (2004) Polyphasic detection of cyanobacteria in terrestrial biofilms. Biofouling 20:71–79
Gaylarde PM, Crispim CA, Neilan BA, Gaylarde CC (2005) Cyanobacteria from Brazilian building walls are distant relatives of aquatic genera. OMICS 9:30–42
Gaylarde CC, Ortega-Morales BO, Bartolo-Perez P (2007) Biogenic black crusts on buildings in unpolluted environments. Curr Microbiol 54:162–166
Heyrman J, Swings J (2001) 16S rDNA Sequence analysis of bacterial isolates from biodeteriorated mural paintings in the Servilia tomb (Necropolis of Carmona, Seville, Spain). Syst Appl Microbiol 24:417–422
Kiel G, Gaylarde C (2006) Bacterial diversity in biofilms on external surfaces of historic buildings in Porto Alegre. World J Microbiol Biotechnol 22:293–297
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Laiz L, Piñar G, Lubitz W, Saiz-Jimenez C (2003) Monitoring the colonization of monuments by bacteria: cultivation versus molecular methods. Environ Microbiol 5:72–74
Lamenti G, Tiano P, Tomaselli L (2000) Biodeterioration of ornamental marble statues in the Boboli Gardens (Florence, Italy). J Appl Phycol 12:427–433
Lan W, Li H, Wang W-D, Katayama Y, Gu J-D (2010) Microbial community analysis of fresh and old microbial biofilms on Bayon Temple Sandstone of Angkor Thom, Cambodia. Microb Ecol 60:105–115
McNamara CJ, Perry TD, Bearce KA, Hernandez-Duque G, Mitchell R (2006) Epilithic and endolithic bacterial communities in limestone from a Maya archaeological site. Microbial Ecol 51:51–64
McSpadden Gardener BB (2004) Ecology of Bacillus and Paenibacillus spp. in agricultural systems. Phytopathology 94:1252–1258
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Ortega-Morales O, Guezennec J, Hernandez-Duque G, Gaylarde CC, Gaylarde PM (2000) Phototrophic biofilms on ancient Mayan buildings in Yucatan, Mexico. Curr Microbiol 40:81–85
Ortega-Morales BO, Narvaez-Zapata JA, Schmalenberger A, Dousa-Lopez A, Tebbe CC (2004) Biofilms fouling ancient limestone Mayan monuments in Uxmal, Mexico: a cultivation-independent analysis. Biofilms 1:79–90
Peraza Zurita Y, Cultrone G, Sánchez Castillo P, Sebastián E, Bolívar FC (2005) Microalgae associated with deteriorated stonework of the fountain of Bibatauín in Granada, Spain. Int Biodeterior Biodegr 55:55–61
Rosselló-Mora R, Amann R (2001) The species concept for prokaryotes. FEMS Microbiol Rev 25:39–67
Sanguinetti CJ, Neto ED, Simpson AJG (1994) Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 17:914–921
Scheffer M, Rinaldi S, Gragnani A, Mur LR, van Nes EH (1997) On the dominance of filamentous cyanobacteria in shallow, turbid lakes. Ecology 78:272–282
Smerda J, Sedlácek I, Pacova Z, Krejcí E, Havel L (2006) Paenibacillus sepulcri sp. nov., isolated from biodeteriorated mural paintings in the Servilia tomb. Int J Syst Evol Microbiol 56:2341–2344
Valério E, Faria N, Paulino S, Pereira P (2008) Seasonal variation of phytoplankton and cyanobacteria composition and associated microcystins in six Portuguese freshwater reservoirs. Ann Limnol 44:189–196
Walker JJ, Pace NR (2007) Phylogenetic composition of rocky mountain endolithic microbial ecosystems. Appl Environ Microbiol 73:3497–3504
Warscheid T (2000) Integrated concepts for the protection of cultural artefacts against biodeterioration. In: Of microbes and art: the role of microbial communities in the degradation and protection of cultural heritage. Kluwer, Amsterdam, pp 185–201
Warscheid T, Leisen H (2011) Microbiological studies on stone deterioration and development of conservation measures at Angkor Wat. In: Charola AE, McNamara C, Koestler RJ (eds) Biocolonization of stone: control, preventive methods. Smithsonian Inst, Washington, pp 1–18
Wee YC, Lee KB (1980) Proliferation of algae on surfaces of buildings in Singapore. Int Biodeter Bull 16:113–117
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaylarde, C.C., Rodríguez, C.H., Navarro-Noya, Y.E. et al. Microbial Biofilms on the Sandstone Monuments of the Angkor Wat Complex, Cambodia. Curr Microbiol 64, 85–92 (2012). https://doi.org/10.1007/s00284-011-0034-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-011-0034-y