Abstract
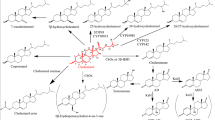
Compactin, a hypocholesterolemic molecule, is a competitive inhibitor of 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, which is a regulatory enzyme for cholesterol biosynthesis. The structural similarity and high affinity of the acid form of compactin and HMG, the natural substrate of enzyme, results in specific and effective inhibition of this enzyme. Inhibition results in reduced levels of mevalonic acid in the body, leading to pleiotropic effects. Various fungi have been used for the commercial production of compactin. Using different strategies for improving production levels, yields have been increased to around 900 times of the amount originally produced. Recently, the gene sequence responsible for compactin production has been cloned and sequenced. This review deals with the chemistry, mode of action, pharmacology, biosynthesis, and production of compactin. A comparative study of various reports dealing with the production of compactin is also included.

Similar content being viewed by others
References
Abe Y, Suzuki T, Ono C, Iwamoto K, Hosobuchi M, Yoshikawa H (2002a) Molecular cloning and characterization of an ML-236B (compactin) biosynthetic gene cluster in Penicillium citrinum. Mol Genet Genomics 267:636–646
Abe Y, Suzuki T, Mizuno T, Ono C, Iwamoto K, Hosobuchi M, Yoshikawa H (2002b) Effects of increased dosage of the ML-236B (compactin) biosynthetic gene cluster on ML-236B production in Penicillium citrinum. Mol Genet Genomics 268:130–137
Albers-Schonberg, Joshou H, Lopez MB (1981) Hypocholesteremic fermentation products and products of preparation. US Patent 4 294 846
Alberts AW (1998) Discovery, biochemistry and biology of lovastatin. Am J Cardiol 62:10J–15J
Amin-Hanjani S, Stagliano NE, Yamada M, Huang PL, Liao JK, Mosokowitz MA (2001) Mevastatin, an HMG-CoA reductase inhibitor, reduces stroke damage and upregulates endothelial nitric oxide synthase in mice. Stroke 32:980–986
Askenazi M, Driggers EM, Holtzman DA, Norman TC, Iverson S, Zimmer DP, Boers ME, Blomquist PR, Martinez EJ, Monreal AW, Feibelman TP, Mayorga ME, Maxon ME, Sykes K, Tobin JV, Cordero E, Salama SR, Trueheart J, Royer JC, Madden KT (2003) Integrating transcriptional and metabolite profiles to direct the engineering of lovastatin-producing fungal strains. Nat Biotechnol 21:150–156
Bazaraa WA, Hamdy MK, Toledo R (1998) Bioreactor for continuous synthesis of compactin by Penicillium cyclopium. J Ind Microbiol Biotechnol 2:192–202
Beg ZH, Stonik JA (1982) Reversible inactivation of 3-hydroxy-3-methylglutaryl coenzyme A reductase: reductase kinase and meavlonate kinase are separate enzymes. Biochem Biophys Res Commun 108:559–566
Bowmen L, Geiger E (1984) Optimization of fermentation conditions for alcohol production. Biotechnol Bioeng 26:1492–1497
Brown AG, Smale TC, King J, Hasenkamp R, Thompson RH (1976) Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J Chem Soc Perkin 1:1165–1170
Brown MS, Faust JR, Goldstein JL (1978) Induction of 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in human fibroblasts incubated with compactin (ML-236B), A competitive inhibitor of the reductase. J Biol Chem 253:1121–1128
Byrne GS, Ward OP (1989) Effect of nutrition on pellet formation by Rhizopus arrhizus. Biotechnol Bioeng 33:912–914
Chakravarti R, Sahai V (2002a) A chemically-defined medium for production of compactin by Penicillium citrinum. Biotechnol Lett 24:527–530
Chakravarti R, Sahai V (2002b) Optimisation of compactin production in chemically defined production medium by Penicillium citrinum using statistical methods. Process Biochem 38:481–486
Chung KJ, Lee JK, Park JW, Seo DJ, Lee SC (2001) Method for producing pravastatin precursor, ML-236B. US Patent 6 204 032
Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F (1999) New insights into the pharmacodynamics and pharmokinetics properties of statins. Pharmacol Theraput 84:413–428
Davidson MH, Jacobson TA (2001) How statins work: the development of cardiovascular diseases and its treatment with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. www.medscape.com/viewprogram/608
Davignon J, Mabile L (2001) Mechanisms of action of statins and their pleiotropic effects. Ann Endocinol 62:101–112
Davis BL, Cino PM, Szarka L (2000) Enzymatic hydroxylation process for the preparation of HMG-CoA reductase inhibitors and intermediates thereof. US Patent 6 043,064
Demain AL, Kennel YM, Aharonowitz Y (1979) Carbon catabolic regulation of secondary metabolism. Microbial technology: current status, future prospects. Symp Soc Gen Microbiol 29:168–185
Demain AL, Peng Y, Yashphe J, Davis J (1999) Conversion of compactin to pravastatin by Actinomadura. US Patent 5 942 423
Demain AL, Peng Y, Yashphe J, Davis J (2001) Conversion of compactin to pravastatin by Actinomadura. US Patent 6 274 360
Dey G, Mitra A, Banerjee R, Maiti BR (2001) Enhanced production of amylase by optimization of nutritional constituents using response surface methodology. Biochem Eng J 7:227–231
Endo A (1985) Compactin (ML-236 B) and related compounds as potential cholesterol lowering agents that HMG-CoA reductase. J Med Chem 28:400–405
Endo A, Yamashita H (1985) Microbial phosphorylation of compactin (ML-236B) and related compounds. J Antibiot 38:328–332
Endo A, Kuroda M, Tsujita Y (1976a) ML-236A, ML-236B and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinum. J Antibiot 29:1346–1348
Endo A, Kuroda M, Tanzawa K (1976b) Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B, fungal metabolites, having hypocholesterolemic activity. FEBS Lett 72:323–326
Endo A, Kuroda M, Terahara A, Yoshio T, Chhiro T (1977) Physiologically active substances and fermentative process for producing the same. US Patent 4049495
Endo A, Hasumi K, Negishi S (1985a) Monacolins J and L, new inhibitors of cholesterol biosynthesis produced by Monascus rubber. J Antibiot 38:420–423
Endo A, Negishi Y, Iwashita T, Mizukawa K, Hirama M (1985b) Biosynthesis of ML-236B (compactin) and monacolin K. J Antibiot 38:444–448
Endo A, Hasumi K, Yamada A, Shimoda R, Takeshima H (1986) The synthesis of compactin (ML-236B) and monacolin K in fungi. J Antibiot 39:1609–1610
Gallo M, Katz E (1972) Regulation of secondary metabolite biosynthesis: catabolite repression of phenoxazine synthase and actinomycin formation by glucose. J Bacteriol 109:659–667
Galloway P, Greer IA, Sattar N (2002) Statins as novel therapies for osteoporosis. Eur J Obstet Gynecol Reprod Biol 101:4–5
George R, Menon AS, Ramasarma T (1980) Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by adenosine derivatives and fatty acylcoenzyme A. Ind J Biochem Biophys 17:1–7
Gerson DF, Xiao X (1995) Process for the production of lovastatin using Coniothyrium fuckelii. US Patent 5 409 820
Hamelin BA, Turgeon J (1998) Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharamcol Sci 19:26–37
Hosobuchi M, Shiori T, Ohyama J, Arai M, Iwado S, Yoshikawa H (1993a) Production of ML-236B, and inhibitor of 3-hydroxy-3-methylglutaryl CoA reductase, by Penicillium citrinum: improvement of strain and culture conditions. Biosci Biotechnol Biochem 57:1414–1419
Hosobuchi M, Ogawa K, Yoshikawa H (1993b) Morphology study in production of ML-236B, a precursor of pravastatin sodium by Penicillium citrinum. J Ferment Bioeng 76:470–475
Hosobuchi M, Fukui F, Matsukawa H, Suzuki T, Yoshikawa H (1993c) Morphology control of preculture during production of pravastatin sodium by Penicillium citrinum. J Ferment Bioeng 76:476–481
Hosobuchi M, Fukui F, Suzuki T, Yoshikawa H (1993d) Fuzzy control in microbial production of ML-236B, a precursor of pravastatin sodium. J Ferment Bioeng 76:482–486
Hutchinson CR, Kennedy J, Park C, Kendrew S, Auclair K, Vederas J (2000) Aspects of the biosynthesis of non-aromatic fungal polyketide by iterative polyketide synthases. Antonie van Leeuwenhoek 78:287–295
Kalil SJ, Maugeri F, Rodrigues MI (2000) Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem 35:539–550
Koizumi J, Mabuchi H, Takeda R (1982) A possible translational control of 3-hydroxy-3-methylglutaryl coenzyme A reductase induction by ML-236B (compactin) in isolated rat hepatocytes. Biochem Biophys Res Commun 108:240–246
Komagata D Yamashita H, Endo A (1986) Microbial conversion of compactin (ML-236B) to ML-236A. J Antibiot 39:1574–1577
Konya A, Jekkel A, Suto J, Salat J (1998) Optimization of compactin fermentation. J Ind Microbiol Biotechnol 20:150–152
Kranjc S, Ivnac I, Schauer M (2002) Biotechnological process for preparing hydroxylated ML-236B derivatives, known as M-4 and M-4′, and analogues thereof. US Patent 6 365 382
Kumar MS, Jana SK, Senthil V, Shasshanka S, Kumar V, Sadhukhan AK (2000) Repeated fed-batch process for improving Lovastatin production. Process Biochem 36:363–368
Manzoni M, Bergomi S, Rollni M, Cavazzoni V (1999) Production of statins by filamentous fungi. Biotechnol Lett 21:253–257
Martin JF, Revilla G, Zanca DM, Lopez-Nieto MJ (1982) Carbon catabolite regulation of penicillin and cephalosporin biosynthesis. In: Umezawa H et al (eds) Proceedings of symposium Trends in Antibiotic Research. Japan Antibiotic Research Association, Tokyo, pp 258–268
Metz B, Kossen NWF, Van Suizdum JC (1979) The rheology of mould suspensions. Adv Biochem Eng 11:103–156
Miller TL, Wolin MJ (2001) Inhibition of growth of methane-producing bacteria of the ruminant forestomach by hydroxymethylglutaryl-ScoA reductase inhibitors. J Dairy Sci 84:1445–1448
Mok T, Koehler AP, Yu MY, Ellis DH, Johnson PJ, Wickhan NW (1997) Fatal Penicillium citrinum pneumonia with pericarditis in a patient with acute leukemia. J Clin Microbiol 35:2654–2656
Morikawa S, Umetani M, Nakagawa S, Yamazaki H, Suganami H, Inoue K, Kitahara M, Hamakubo T, Kodama T, Saito Y (2000) Relative induction of mRNA for HMG-CoA reductase and LDL receptor by five different HMG-CoA reductase inhibitors in cultured human cells. J Atheroscler Thromb 7:138–144
Nakanishi M, Goldstein JL, Brown MS (1988) Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J Biol Chem 263:8929–8937
Narang S, Sahai V, Bisaria VS (2001) Optimization of xylanase production by Melanocarpus albomyces IIS68 in solid state fermentation using the response surface methodology. J Biosci Bioeng 9:425–427
Papagianni M, Mattey M, Kristiansen B (1994) Morphology and citric acid production of Aspergillus niger PM1. Biotechnol Lett 9:929–934
Paul GC, Priede MA, Thomas CR (1999) Relationship between morphology and citric acid production in submerged Aspergillus niger fermentation. Biochem Eng J 3:121–129
Peng Y, Demain AL (1999) A new hydroxylase system in Actinomadura sp. cells converting compactin to pravastatin. J Ind Microbiol Biotechnol 20:373–375
Peng Y, Yashbe J, Demain AL (1997) Biotransformation of compactin to pravastatin by Actinomadura sp. 2966. J Antibiot 52:1032–1035
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Prapulla SG, Jacob Z, Chand N, Rajalakshmi D, Karanth NG (1992) Maximization of lipid production by Rhodoterula gracilis CFR-1 using the response surface methodology. Biotechnol Bioeng 40:965–970
Primrose S, King D, Yaworski ED, Radhakrishnan J, He D, Xiao X (1997) Fermentative process for preparation of compactin. US Patent 561173
Ramirez C (1982) Manual and atlas of the Penicillia. Elsevier, Amsterdam, pp 349–351
Reynolds KA, Demain AL (1997) Rapamycin, FK506 and ascomycin related compounds. In: Strohl WR (ed) Biotechnology of Antibiotics, 2nd edn. Dekker, New York, p 497
Roseiro JC, Esgalhado ME, Amaral Collaco MT, Emery AN (1992) Medium development for xanthan production. Process Biochem 27:167–175
Serizawa N, Nakagawa K, Hamano K, Tsujita Y, Terahara A, Kuwano H (1983a) Microbial hydroxylation of ML-236B (compactin) and monacolin K (MB-530 B). J Antibiot 36:604–607
Serizawa N, Nakagawa K, Tsujita Y, Terahara A, Kuwano H (1983b) 3-Hydroxy-ML-236B (3-hydroxy compactin), microbial transformation product of ML-236B (compactin). J Antibiot 36:608–610
Serizawa N, Serizawa S, Nakagawa K, Furuya K, Okazaki T, Terahara A (1983c) Microbial hydroxylation of ML-236B (compactin), studies on microorganisms capable of 3α-hydroxylation of ML-236B. J Antibiot 36:887–891
Serizawa N, Nakagawa K, Tsujita Y, Terahara A, Kuwano H (1983d) 6-hydroxy-iso-ML-236B and ML-236A, microbial transformation products of ML-236B. J Antibiot 36:918–920
Sinensky M, Logel J (1983) Inhibition of degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by mevinolin. J Biol Chem 258:8547–8549
Smith JJ, Lilly MD, Fox RI (1990) The effect of agitation on the morphology and penicillin production of Penicillium chrysogenum. Biotechnol Bioeng 35:1011–1023
Sugiyama M, Kodama T, Koniishi K, Abe K, Asami S, Oikawa S (2000) Compactin and Simvastatin, but not pravastatin, induces bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun 271:688–692
Sutherland A, Auclair K, Vederas (2001) Recent advances in the biosynthetic studies of lovastatin. Curr Opin Drug Discovery Dev 4:229–326
Tanzawa K, Endo A (1979) Kinetic analysis of reaction catalyzed by rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase using two specific inhibitors. Eur J Biochem 98:195–210
Tobert JA (1987) New developments in lipid-lowering therapy: the role of inhibitors of hydroxymethylglutaryl coenzyme A reductase. Circulation 76:534–538
Tony Lam YK, Gullo VP, Goeglman RT, Jorn D, Huang L, Deriso C, Monaghan RL, Putter I (1981) Dihydrocompactin, a new potent inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase from Penicillium citrinum. J Antibiot 34:614–616
Vinci VA, Conder MJ, McAda PC, Phyllis C, Reeves CD, Rambosek J, Davis CR, Hendrickson LE (2001) DNA encoding triol polyketide synthase. US Patent 6 174 706
Wächtershäuser A, Akoglu B, Stein J (2001) HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis 22:1061–1067
Wang YP, Chen YC, Chang W, Lin CL (2001) Mutant strain of Penicillium citrinum and use thereof for preparation of compactin. US Patent 6 323 021
Wasser SP, Reshetnikov SV (2002) Process for producing, methods and compositions of cholesterol lowering agents from higher basidiomycetes mushrooms. US Patent 6 372 462
Yamashita H, Tsubokawa S, Endo A (1985) Microbial hydroxylation of compactin (ML-236B) and monacolin K. J Antibiot 38:605–609
Ykema A, Lindsay JM (2000) Nitrogen feed in statin fermentation. US Patent 6 165 757
Yuki A, Hosobuchi M, Yoshokawa H (2001) Penicillium citrinum genes associated with biosynthesis of ML-236B, precursor of 3-hydroxy-3-methylglutaryl CoA reductase inhibitor. World Patent 0112814
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakravarti, R., Sahai, V. Compactin—a review. Appl Microbiol Biotechnol 64, 618–624 (2004). https://doi.org/10.1007/s00253-003-1553-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1553-7