Abstract
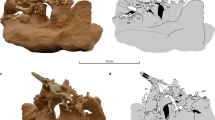
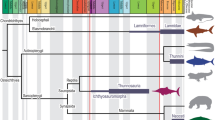
Decompression syndrome (caisson disease or the “the bends”) resulting in avascular necrosis has been documented in mosasaurs, sauropterygians, ichthyosaurs, and turtles from the Middle Jurassic to Late Cretaceous, but it was unclear that this disease occurred as far back as the Triassic. We have examined a large Triassic sample of ichthyosaurs and compared it with an equally large post-Triassic sample. Avascular necrosis was observed in over 15 % of Late Middle Jurassic to Cretaceous ichthyosaurs with the highest occurrence (18 %) in the Early Cretaceous, but was rare or absent in geologically older specimens. Triassic reptiles that dive were either physiologically protected, or rapid changes of their position in the water column rare and insignificant enough to prevent being recorded in the skeleton. Emergency surfacing due to a threat from an underwater predator may be the most important cause of avascular necrosis for air-breathing divers, with relative frequency of such events documented in the skeleton. Diving in the Triassic appears to have been a “leisurely” behavior until the evolution of large predators in the Late Jurassic that forced sudden depth alterations contributed to a higher occurrence of bends.



Similar content being viewed by others
References
Altringham JD, Shadwick RE (2001) Swimming and muscle function. In: Block BA, Stevens ED (eds) Fish physiology, vol 19. Academic, San Diego, pp 313–341
Andersen HT (1996) Physiological adaptations in diving vertebrates. Physiol Rev 46:212–258
Arthur PG, West TG, Brill RW, Schulte PM, Hochachka PW (1992) Recovery metabolism of skipjack tuna (Katsuwonus pelamis) white muscle: rapid and parallel changes on lactate and phosphocreatine after exercise. Can J Zool 70:1230–1239
Beatty B, Rothschild BM (2008) Decompression syndrome and the evolution of deep diving physiology in the Cetacea. Naturwissenschaften 95:793–801
Bennett AF, Hicks JW, Cullum AJ (2000) An experimental test of the thermoregulatory hypothesis for the evolution of endothermy. Evolution 54:1768–1773
Benton MJ (2005) Vertebrate paleontology. Wiley-Blackwell, Oxford
Bernard A, Lécuyer C, Vincent P, Amiot R, Bardet N, Buffetaut E, Cuny G, Fourel F, Martineau F, Mazin J-M, Prieur A (2010) Regulation of body temperature by some Mesozoic marine reptiles. Science 328:1379–1382
Block BA, Finnerty JR, Stewart AF, Kidd J (1993) Evolution of endothermy in fish: mapping physiological traits on a molecular phylogeny. Science 260:210–214
Brill RW, Bushnell PG (2001) The cardiovascular system of tunas. In: Block BA, Stevens ED (eds) Tuna: physiology, ecology and evolution. Fish physiology, vol 19. Academic, San Diego, pp 709–820
Cappetta H (1987) Chondrichthyes II. Mesozoic and Cenozoic Elasmobranchii. Handbook of paleoichthyology 3B. Fischer, Stuttgart
Carey FG, Teal JM, Kanwisher JW, Lawson KD (1971) Warm bodied fish. Amer Zool 11:135–145
Carlson JK, Goldman KJ, Lowe CG (2004) Metabolism, energetic demand, and endothermy. In: Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Boca Raton, pp 203–224
De Buffrénil V, Mazin J (1990) Bone histology of the ichthyosaurs: comparative data and functional interpretation. Paleobiology 16:435–447
Feldman JL, Menkes CJ, Amor B, Cherot A, Delbvarr FL (1981) Osteonecrosis vertebrale de l’adults. Rev Rhum 48:773–780
Forsman ED, Otto IA (2006) Healed fractures and other abnormalities in bones of small mammals. Northwest Nat 87:143–146
Frid A, Heithaus MR, Dill LM (2007) Dangerous dive cycles and the proversial ostrich. Oikos 116:893–902
Graham JB, Dickson KA (2000) The evolution of thunniform locomotion and heat conservation in scombrid fishes: new insights based on the morphology of Allothunnus fallai. Zool J Linnean Soc Lond 129:419–466
Graham JB, Dickson KA (2001) Anatomical and physiological specializations for endothermy. In: Block BA, Stevens ED (eds) Tuna: physiology, ecology and evolution. Fish physiology, vol 19. Academic, San Diego, pp 121–165
Graham JB, Dickson KA (2004) Tuna comparative physiology. J Exp Biol 207:4015–4024
Heinrich B (1989) Beating the heat in obligate insect endotherms: the environmental problem and the organismal solutions. Intergr Comp 29:1157–1168
Heithaus MR, Frid A (2003) Optimal diving under the risk of predation. J Theor Biol 223:79–92
Katz SL, Syme DA, Shadwick RE (2001) High-speed swimming: enhanced power in yellowfin tuna. Nature 410:770–771
Keen JW, Aota S, Brill RW, Farrell AO, Randall DJ (1995) Cholinergic and adrenergic regulation of heart rate and ventral aortic pressure in two species of tropical tunas: Katsuwanus pelamis and Thunnus albacares. Can J Zool 73:1681–1688
Kitagawa T, Kimura S, Nakita H, Yamada H (2004) Diving behavior of immature, feeding Pacific bluefin tuna (Thunnus thynnus orientalis) in relation to season and area: the East China Sea and the Kuroshio-Oyashio transition region. Fish Oceanogr 13:161–180
Kooyman GL, Schroeder JP, Green DG, Smith VA (1973) Gas exchange in penguins during simulated dives to 30 and 68 meters. Am J Physiol 225:1467–1471
Lig S, Lrowet K (2010) Timing of deep-sea adaptation in dogfish sharks: insights from a supertree of extinct and extant taxa. Zool Scripta 39:331–342
Lowe TE, Brill RW, Cousins KL (2000) Blood oxygen-binding characteristics of big eye tuna (Thunnus obesus), a high-energy-demand teleost that is tolerant to low ambient oxygen. Mar Biol 136:1087–1098
Maisch MW (2010) Phylogeny, systematics, and origin of the Ichthyosauria—the state of the art. Palaeodiversity 3:151–214
Massare JA (1987) Tooth morphology and prey preference of Mesozoic marine reptiles. J Vert Paleontol 7:121–137
Massare JA, Callaway JM (1990) The affinities and ecology of Triassic ichthyosaurs. Geol Soc Am Bull 102:409–416
Mathie N (2007) New dams may flush bottom-breathers out. Australas Sci. June 2007:36
Mathieu-Costello O, Brill RW, Hochachka PW (1996) Structural basis for oxygen delivery: muscle capillaries and manifolds in tuna red muscle. Comp Biochem Physiol A Physiol 113:25–31
McGowan C (1972) The distinction between latipinnate and longipinnate ichthyosaurs. Life Sci Occas Paper 20:1–8
McGowan C, Motani R (2003) Ichthyopterygia. Handbook of paleoherpetology, vol 8. Friedrich Pfeil, Munchen, pp 1–175
Michel JL, Bouzat J, Rivoal A, Pradel D, de Lamaze PH, Viallet JF, Belin J, Merle P (1982) La dissection gazeuse du corps vertebral ou phenomene du vide intrasomatique vertebral. J Radiol 63:479–484
Motani R (2002) Swimming speed estimation of extinct marine reptiles: energetic approach revisited. Paleobiology 28:251–262
Motani R (2010) Warm-blooded “sea dragons”? Science 328:1361–1362
Motani R, Rothschild BM, Wahl W Jr (1999) Large eyeballs in diving ichthyosaurs. Nature 402:747
Pauley P (1965) Decompression sickness following repeated breath-hold dives. J Appl Physiol 20:1028–1031
Ratcliffe JF (1985) Anatomic basis for the pathogenesis and radiologic features of vertebral osteomyelitis and its differentiation from childhood discitis: a microarteriographic investigation. Acta Radiol 26:137–143
Resnick D (2002) Diagnosis of bone and joint disorders. Saunders, Philadelphia
Rossi-Fanelli A, Antonini E (1960) Oxygen equilibrium of hemoglobin from Thunnus thynnus. Nature 186:895–896
Rothschild BM (1982) Rheumatology: a primary care approach. Yorke Medical Press, New York
Rothschild BM (1987) Decompression syndrome in fossil marine turtles. Ann Carnegie Mus 56:253–358
Rothschild BM (1990) Absence of decompression syndrome in recent and fossil Mammalia and Reptilia. Ann Carnegie Mus 59:287–293
Rothschild BM (2011) Pathology in saber tooth ecomorphs. In: Naples V, Martin LD, Babiarz J (eds) Saber-toothed cats. John Hopkins Press, Baltimore, pp 35–41
Rothschild BM, Martin LD (1987) Avascular necrosis: occurrence in diving Cretaceous mosasaurs. Science 236:75–77
Rothschild BM, Martin LD (2006) Skeletal impact of disease. New Mexico Museum of Natural History, Albuquerque
Rothschild BM, Storrs GW (2003) Decompression syndrome in plesiosaurs (Sauropterygia: Reptilia). J Vertebr Paleontol 23:324–328
Sander PM, Chen X, Cheng L, Wang X (2011) Short-snouted toothless ichthyosaur from China suggests Late Triassic diversification of suction feeding ichthyosaurs. PLoS One 6(5):e19480. doi:10.1371/journal.pone.0019480
Schultz AH (1969) The life of primates. Unwin Brothers, Ltd, Woking
Strauss MB (1970) Physiological aspects of mammalian breath-hold diving: a review. Aerospace Med 41:1362–1381
Strauss MB, Sampson RL (1986) Decompression syndrome: an update. Phys Sports Med 14(3):1–9
Syme DA, Sjadwick RE (2002) Effects of longitudinal body position and swimming speed on mechanical power of deep red muscle from skipjack tuna (Katsuwonus pelamis). J Exp Biol 205:189–200
Vann RD, Butler FK, Mitchell SJ, Moon RE (2011) Decompression illness. Lancet 377:153–164
Acknowledgments
We express appreciation to Dave Burnham, Eugene Gaffney, Chun Li, Judy Massare; Desui Miao; Frank Rühli and William Sampson for access to collections that they curate and to cogent manuscript review and commentary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Rothschild, B.M., Xiaoting, Z. & Martin, L.D. Adaptations for marine habitat and the effect of Triassic and Jurassic predator pressure on development of decompression syndrome in ichthyosaurs. Naturwissenschaften 99, 443–448 (2012). https://doi.org/10.1007/s00114-012-0918-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-012-0918-0