Abstract
Background
Anti-inflammatory and antioxidative potential of hesperidin and naringin was carried out considering the rat air pouch model of inflammation.
Methods
Reference dose of hesperidin (H) or naringin (N) or indomethacin (I) was administered to the rat air pouches. The pouches were induced by injecting sterile air into the intra-scapular region of the rats followed by carrageenan (AP + C) administration. Rats injected only with air (AP) served as controls.
Results
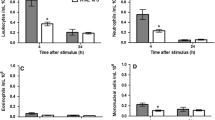
The AP + C group, showed an increase in the exudate lipid peroxidation (LPO), reduced glutathione (GSH), TNF-α, activity of catalase (CAT), total leukocytes and neutrophils along with tissue edema and infiltration of inflammatory cells. Increases in tissue nitrite, LPO, GSH, SOD (superoxide dismutase) and CAT were recorded. Increased CAT and SGPT with concomitant decrease in ALP were observed in serum. When treated with indomethacin (AP + C + I), all the alterations in the exudate, tissue and serum shifted towards normalcy, except LPO in exudate and nitrite in tissue, while, hesperidin (AP + C + H) or naringin (AP + C + N) treatment normalized all the alterations.
Conclusion
It seems that both naringin and hesperidin are anti-inflammatory and antioxidative in nature, but hesperidin proved to be better than indomethacin and naringin because of more pronounced pharmacological actions without tissue toxicity.

Similar content being viewed by others
References
Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Park YB, Rhee SJ, Choi MS. Antioxidative activity of naringin and lovastin in high cholesterol-fed rabbits. Life Sci. 2001;69(24):2855–66.
Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Jeong TS, Park YB, Choi MS. Comparison of naringin and probucol in cholesterol-fed rabbits. Clin Chem Acta. 2002;317(1–2):181–90.
Jeon SM, Park YB, Choi MS. Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr. 2004;23(5):1025–34.
Chen MC, Ye YY, Ji G, Liu JW. Hesperidin upregulates heme oxyenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J Agric Food Chem. 2010;58(6):3330–5.
Zielinska-Przyiemska M, Ignatowicz E. Citrus fruit flavanoids influence on neutrophil apoptosis and oxidative metabolism. Phytother Res. 2008;22(12):1557–62.
Yeh MH, Kao ST, Hung CM, Liu CJ, Lee KH, Yeh CC. Hesperidin inhibited acetaldehyde-induced matrix metalloproteinase-9 gene expression in human hepatocellular carcinoma cells. Toxicol Lett. 2009;184(3):204–10.
Emim JA, Oliveria AB, Lapa AJ. Pharmacological evaluation of the ant-inflammatory activity of a citrus bioflavonoid, hesperidin and the isoflavonoids, duartin and claussequinone in rats and mice. J Pharm Pharmacol. 1994;46(2):118–22.
Guardia T, Rotelli AE, Juarez AO, LE Pelzer. Anti-inflammatory properties of plant flavonoids. Effect of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683–7.
Ribeiro IA, João R, Bruno S, Mota-Filipe H, Ribeiro HM. Effect of naringin enzymatic hydrolysis towards naringenin on the anti-inflammatory activity of both compounds. J Mol catal B Enzym. 2008;52(53):13–8.
Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new finding in anticancer, cardiovascular and anti-inflammatory activity. J Agric Food Chem. 2008;56(15):6185–205.
Liu L, Shan S, Zhang K, Ning ZQ, Lu XP, Cheng Y. Naringenin and hesperitin, two flavonoids derived from Citrus aurrantium up-regulate transcription of adiponectin. Phytother Res. 2008;22(10):1400–3.
Leontowicz H, Gorinstein S, Lojek A, Leontowicz M, Ciz M, Soliva-Fortuny R, Park YS, Jung ST, Trakhtenberg S, Martin-Belloso O. Comparative content of some bioactive compounds in apples, peaches and pears and their influence on lipids and antioxidant capacity in rats. J Nutr Biochem. 2002;13(10):603–10.
Parmar HS, Kar A. Comparative analysis of free radical scavenging potential of several fruit peel extracts by in vitro method. Drug Discov Ther. 2009;3(2):49–55.
Morikawa K, Nonaka M, Narahara M, Torii I, Kawaguchi K, Yoshikawa T, Kumazawa Y, Morikawa S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life sci. 2003;74(6):709–21.
Sedgwick AD, Lees P. Studies of eicosanoid production in the air pouch model of synovial inflammation. Agents Actions. 1986;18(3–4):429–38.
Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci. 1994;91(8):3228–32.
Kumar P, Kumar A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: possible role of nitric oxide. Behav Brain Res. 2006;206(1):38–46.
Kanno S, Shouji A, Tomizawa A, Hiura T, osanai Y, Ujibe M, Obara Y, Nakahata N, Ishikawa M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006;78(7):673–81.
Bauerova K, Nosalova V, Mihalova D, Bauero JN. Contribution to safe anti-inflammatory therapy with indomethacin. Cent Eur J Public Health. 2004;12:S8–10.
Letelier ME, Molina-Berrios A, Cortes-Tronsco J, Jara-sandoval J, Holst M, Palma K, Montoya M, Miranda D, Gonzalez-Lira V. DPPH and oxygen free radicals as pro-oxidant of biomolecules. Toxicol In Vitro. 2008;22(2):279–86.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;7(72):248–54.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reactions. Anal Biochem. 1979;95(2):451–8.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47(3):469–74.
Aebi HU. Catalase: methods in enzymatic analysis. New York: Academic Press; 1983. vol. 3, p. 276–86.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Clin Pathol. 1957;28(1):56–63.
Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with five cubic millimetres of serum. J Biol Chem. 1946;164:321–9.
Bansal AK, Trivedi R, Soni GL, Bhatnagar D. Hepatic and renal oxidative stress in acute toxicity of N-nitrosodiethylamine in rats. Ind J Exp Biol. 2009;38(9):916–20.
Tanas S, Odabasoglu F, Halici Z, Cakir A, Aygun H, Aslan A, Suleyman H. Evaluation of anti-inflammatory and antioxidant activities of Peltigera rufescens lichen species in acute and chronic inflammation models. J Nat Med. 2010;64(1):42–9.
Ceriello A, Kumar S, Piconi L, Esposito K, Giugliano D. Simultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetes. Diabetes Care. 2007;30(3):649–54.
Griffiths RJ. Prostaglandins and inflammation. In: Gallin JI, Snyderman R, editors: Inflammation: basic principles and clinical correlates. Philadelphia: Lippincott; 1999. p. 349–60.
Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12.
Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract. 2006;12(S1):60–2.
Baiyr Y, Odabasoglu F, Cakir A, Aslan A, Suleman H, Halici M, Kazaz C. The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine. 2006;13(8):584–90.
Laurila JP, Laatikainen LE, Castellone MD, Laukkanen MO. SOD3 Reduces Inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One. 2009;4(6):e5786.
Kanno S, Shouji A, Tomizawam A, Hiura T, Osanai Y, Ujibe M, Obara Y, Nakahata N, Ishikaw M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006;78(7):673–81.
Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Cacinogenesis. 2006;27(6):1257–65.
Kirkova M, Kassabova T, Russanov E. In vivo effects of indomethacin-I activity of anti-oxidant enzymes and lipid peroxidation. Gen Pharmacol. 1992;23(3):503–7.
Deepa PR, Varlakshmi P. Protective effects of certoparin sodium, a low molecular weight heparin derivative, in experimental atherosclerosis. Clin Chim Acta. 2004;339(1–2):105–15.
Gallo RL, Dorscnner RA, Takashima S, Klagsburn M, Eriksson E, Bernfield M. Endothelial cell surface alkaline phosphatase activity is induced by IL-6 released during wound repair. J Invest Dermatol. 1997;109(4):597–603.
Ahmadi A, Hosseinimehr SJ, Naghshvar F, Hajir E, Ghahremani M. Chemoprotective effects of hesperidin against genotoxicity induced by cyclophosphamide in mice bone marrow cells. Arch Pharm Res. 2008;31(6):794–7.
Kazlowaska K, Hsu T, Hou CC, Yang WC, Tsai GJ. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J Ethnopharmacol. 2010;128(1):123–30.
Kaur G, Tirkey N, Chopra K. Beneficial effect of hesperidin on lipopolysaccharide-induced hepatotoxicity. Toxicology. 2006;226(2–3):152–60.
Aggarwal A, Gaur V, Kumar A. Nitric oxide mechanism in the protective effect of naringin against post-stroke depression (PSD) in mice. Life Sci. 2010;86(25–26):928–35.
Acknowledgments
The authors acknowledge the facilities of the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi (DBT) under its M.Sc. Biotechnology programme and the distributed Bioinformatics sub centre. We also thank Dr. Anil Kumar, Head School of Biotechnology for his kind support.
Conflict of interest
Both the authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Jain, M., Parmar, H.S. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm. Res. 60, 483–491 (2011). https://doi.org/10.1007/s00011-010-0295-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0295-0