Abstract
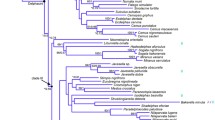
The phylogeny of theDrosophila hydei subgroup, which is a member of theD. repleta species group, was inferred from 1,515 base pairs of mitochondrial DNA sequence of the cytochrome oxidase subunits I, II, and III. Four of the seven species in the subgroup were examined, which are placed into two taxonomic complexes: theD. bifurca complex (D. bifurca) andD. nigrohydei) and theD. hydei complex (D. hydei and (D. eohydei). Both complexes appear to be monophyletic, although theD. bifurca complex is only weakly supported. The evolution of chromosomal change, interspecific crossability, sperm gigantism, and divergence times of the subgroup is discussed in a phylogenetic context.
Similar content being viewed by others
References
Beckenbach AT, Wei YW, Liu H (1993) Relationships in theDrosophila obscura species group, inferred from mitochondrial cytochrome oxidase II sequences. Mol Biol Evol 10:619–634
Beverley SM, Wilson AC (1984) Molecular evolution inDrosophila and the higher Diptera. II. A time scale for fly evolution. J Mol Evol 21:1–13
Brown WM, Prager EM, Wang A, Wilson AC (1982) Mitochondrial DNA sequences of primates: tempo and mode of evolution. J Mol Evol 18:225–239
Casanova J-L, Pannetier C, Jaulin C, Kourilsky P (1990) Optimal conditions for directly sequencing double-stranded PCR products with Sequenase. Nucleic Acids Res 18:4028
Clary DO, Wolstenholme DR (1985) The mitochondrial DNA molecule ofDrosophila yakuba: nucleotide sequence, gene organization and genetic code. J Mol Evol 22:252–271
de Bruijn MHL (1983)Drosophila melanogaster mitochondrial DNA, a novel gene organization and genetic code. Nature 304:234–241
DeSalle R, Freedman T, Prager EM, Wilson AC (1987) Tempo and mode of sequence evolution in mitochondrial DNA of HawaiianDrosophila. J Mol Evol 26:157–164
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Felsenstein J (1993) PHYLIP: Phylogeny inference Package (ver 3.5c). University of Washington, Seattle
Grimaldi DA (1987) Amber fossil Drosophilidae (Diptera), with particular reference to the Hispaniolan taxa. Am Mus Nov 2880:1–23
Hasegawa M, Kishino H, Yano T (1985) Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Hendy MD, Penny D (1982) Branch and bound algorithms to determine minimal evolutionary trees. Math Biosci 59:277–290
Hihara F, Kurokawa H (1987) The sperm length and the internal reproductive organs ofDrosophila with special references to phylogenetic relationships. Zool Sci (Tokyo) 4:167–174
Hillis DM, Bull JJ (1993) An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol 42:182–192
Jin L, Nei M (1990) Limitations of the evolutionary parsimony method of phylogenetic analysis. Mol Biol Evol 7:82–102
Joly D, Bressac C (1994) Sperm length in Drosophilidae (Diptera): estimation by testis and receptacle lengths. Int J Insect Morphol Embryol 23:85–92
Jukes TH, Cantor CR (1969) Evolution of protein molecules, In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–123
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Lachaise D, Carion M-L, David JR, Lemeunier F, Tsacas L, Ashburner M (1988) Historical biogeography of theDrosophila melanogaster species subgroup. Evol Biol 22:159–225
Lake JA (1994) Reconstructing evolutionary trees from DNA and protein sequences: paralinear distances. Proc Natl Acad Sci USA 91: 1455–1459
Liu H, Beckenbach AT (1992) Evolution of the mitochondrial cytochrome oxidase II gene among 10 orders of insects. Mol Phyl Evol 1:41–52
Lockhart PJ, Steel MA, Hendy MD, Penny D (1994) Recovering evolutionary trees under a more realistic model of sequence evolution. Mol Biol Evol 11:605–612
Maddison WP, Maddison DR (1992) MacClade: analysis of phylogeny and character evolution (ver 3.05). Sinauer, Sunderland, MA
Margush T, McMorris FR (1981) Consensus n-trees. Bull Math Biol 43:239–244
Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich HA (1987) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51:263–273
Pitnick S (1996) Investment in testes and the cost of making long sperm inDrosophila. Am Nat 148:57–80
Pitnick S, Markow TA (1994) Large-male advantages associated with costs of sperm production inDrosophila hydei, a species with giant sperm. Proc Natl Acad Sci USA 91:9277–9281
Pitnick S, Markow TA, Spicer GS (1995a) Delayed male maturity is a cost of producing large sperm inDrosophila. Proc Natl Acad Sci USA 92:10614–10618
Pitnick S, Spicer GS, Markow TA (1995b) How long is a giant sperm? Nature 375:109
Rohlf FJ (1982) Consensus indices for comparing classifications. Math Biosci 59:131–144
Russo CAM, Takezaki N, Nei M (1995) Molecular phylogeny and divergence times of Drosophilid species. Mol Biol Evol 12:391–404
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239: 487–491
Saitou N, Imanishi M (1989) Relative efficiencies of the Fitch-Margolish, maximum-parsimony, maximum-likelihood, minimum-evolution, and neighbor joining methods of phylogenetic tree reconstruction in obtaining the correct tree. Mol Biol Evol 6:514–525
Schäfer U (1978) Sterility inDrosophila hydei ×D. neohydei hybrids. Genetica 49:205–214
Simon C, Franke A, Martin A (1991) The polymerase chain reaction: DNA extraction and amplification. In: Hewitt GM (ed) Molecular techniques in taxonomy. NATO Advanced Studies Institute, H57, Springer Berlin, pp 329–355
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
Sokal RR, Sneath PHA (1963) Principles of numerical taxonomy. W.H. Freeman, San Francisco
Spicer GS (1988) Molecular evolution among someDrosophila species as indicated by two-dimensional electrophoresis. J Mol Evol 27: 250–260
Spicer GS (1995) Phylogenetic utility of the mitochondrial cytochrome oxidase gene: molecular evolution of theDrosophila buzzattii species complex. J Mol Evol 41:749–759
Swofford DL (1996) PAUP*Star (test ver 4.0). Sinauer, Sunderland, MA
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Villela CR (1983) A revision of theDrosophila repleta group (Diptera: Drosophilidae). Rev Bras Entomol 27:1–114
Wasserman M (1954) Cytological studies of the repleta group. Univ Texas Publ 5422:130–152
Wasserman M (1962) Cytological studies of the repleta group of the genus Drosophila. IV. The hydei subgroup. Univ Texas Publ 6205: 73–84
Wasserman M (1982) Evolution of therepleta group. In: Ashburner M, Carson HL, Thompson JN (eds) The genetics and biology ofDrosophila, vol 3B. Academic Press, London, pp 61–139
Wasserman M (1992) Cytological evolution of theDrosophila repleta species group. In: Krimbas CB, Powell JR (eds)Drosophila inversion polymorphism. CRC Press, Boca Raton, pp 455–552
Wharton LT (1944) V. Interspecific hybridization in the repleta group. Univ Texas Publ 4445:175–193
Wheeler MR (1949) XIII. Taxonomic studies on the Drosophilidae. Univ Texas Pub 4920:157–195
Yang Z (1994) Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol 39:306–314
Author information
Authors and Affiliations
Additional information
Correspondence to: G. Spicer
Rights and permissions
About this article
Cite this article
Spicer, G.S., Pitnick, S. Molecular systematics of theDrosophila hydei subgroup as inferred from mitochondrial DNA sequences. J Mol Evol 43, 281–286 (1996). https://doi.org/10.1007/BF02338836
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02338836