High genetic diversity and mixing of coastal horseshoe crabs (Tachypleus gigas) across major habitats in Sundaland, Indonesia
- Published
- Accepted
- Received
- Academic Editor
- James Reimer
- Subject Areas
- Biodiversity, Biogeography, Conservation Biology, Ecology, Marine Biology
- Keywords
- Population genetics, Zoogeography, Biogeography, Molecular biology, Endangered species, Protected species, Coral triangle, Population structure, Meta-population
- Copyright
- © 2021 Aini et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. High genetic diversity and mixing of coastal horseshoe crabs (Tachypleus gigas) across major habitats in Sundaland, Indonesia. PeerJ 9:e11739 https://doi.org/10.7717/peerj.11739
Abstract
Species with limited dispersal abilities are often composed of highly genetically structured populations across small geographic ranges. This study aimed to investigate the haplotype diversity and genetic connectivity of the coastal horseshoe crab (Tachypleus gigas) in Indonesia. To achieve this, we collected a total of 91 samples from six main T. gigas habitats: Bintan, Balikpapan, Demak, Madura, Subang, and Ujung Kulon. The samples were amplified using primers for mitochondrial (mt) AT-rich region DNA sequences. The results showed 34 haplotypes, including seven shared and 22 unique haplotypes, across all localities. The pairwise genetic differentiation (FST) values were low (0 to 0.13) and not significantly different (p > 0.05), except among samples from Ujung Kulon-Madura and Kulon-Subang (p < 0.05). Additionally, the 34 analysis of molecular variance (AMOVA) showed the most variation within populations (95.23%) compared to less among populations (4.77%). The haplotype network showed evidence of shared haplotypes between populations. Tajima’s D and Fu’s FS test values indicated a population expansion. Our results showed a low level of differentiation, suggesting a single stock and high connectivity. Therefore, a regionally-based conservation strategy is recommended for the coastal horseshoe crab in Indonesia.
Introduction
High rates of gene flow are common in marine organisms that are spread across large geographic ranges (Palumbi, 1994; Crandall et al., 2019). Several marine organisms also exhibit low levels of genetic differentiation across large geographic scales (Avise, 2000). Population structures are affected by genetic drift, strong post-settlement selection (Hedgecock, 1986), and spatial-landscape patterns (Johnson & Black, 1998; Watts & Johnson, 2004). Species with limited dispersal abilities are often composed of highly genetically structured populations with small geographic ranges (Collin, 2001). This creates opportunities to compare the depths and positions of intraspecific genetic differentiation when using location as an extrinsic factor (Bernardi & Talley, 2000).
Horseshoe crabs, an interesting group of marine organisms considered “living fossils” (Eldredge & Stanley, 1984), have been extant for almost 500 million years. There are four extant species of horseshoe crabs: the American horseshoe crab (Limulus polyphemus) found along the eastern coast of North America from Maine to Mexico (Walls, Berkson & Smith, 2002; Rutecki, Carmichael & Valiela, 2004), and three Asian horseshoe crabs species (the mangrove horseshoe crab (Carcinoscorpius rotundicauda), the coastal horseshoe crab (Tachypleus gigas), and the tri-spined horseshoe crab (Tachypleus tridentatus)) (John et al., 2018; Vestbo et al., 2018) that are sporadically distributed across Southeast Asia and Japan. They are ancient marine arthropods that exhibit life-histories and habitat preferences that suggest a restricted dispersal ability (Sekiguchi, 1988). The Asian species are found in Indonesian coastal waters, dispersed around Sumatra, Java, Kalimantan, and Sulawesi (Rubiyanto, 2012; Mashar et al., 2017; Meilana et al., 2016).
Throughout their life cycle, horseshoe crabs are highly dependent on environmental conditions in coastal habitats. Most research suggests that they are declining both locally and regionally due to over-harvesting for food and biomedicine, and coastal development (Itow, 1993; Botton, 2001; Chen, Yeh & Lin, 2004) and the loss of suitable spawning grounds. T. gigas was once relatively common along the northern Java Sea. However, coastal and mangrove horseshoe crab populations have an undetermined conservation status due to insufficient data (John et al., 2021). Furthermore, most population genetic studies on horseshoe crabs have focused on the American horseshoe crab, with little attention paid to the Asian horseshoe crab (Pierce, Tan & Gaffney, 2000; King & Eackles, 2004; King et al., 2005; Yang et al., 2007; Rozihan & Ismail, 2011; King et al., 2015). Therefore, this study examined the genetic diversity, connectivity, and population structure of coastal horseshoe crabs by screening an AT-rich region of mitochondrial DNA, an established genetic marker for arthropods (Brehm et al., 2001). Our aim was to use genetic evidence to facilitate horseshoe crab conservation efforts in Indonesia.
Materials & Methods
Study area and sample collection
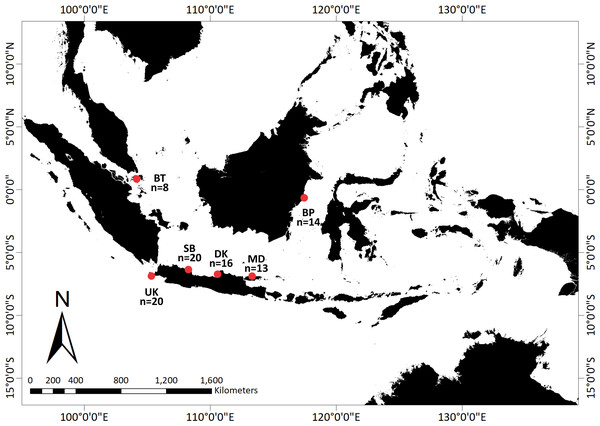
With the help of a local fisherman, adult and juvenile T. gigas specimens were collected from shallow waters in six locations around Indonesia: Bintan, Balikpapan, Demak, Madura, Subang, and Ujung Kulon (Fig. 1). We collected the hemolymph from a total of 91 T. gigas specimens between April 2019 and August 2020. There were eight, 14, 16, 13, 20, and 20 samples from Bintan Island (BT), Balikpapan (BP), Demak (DK), Madura (MD), Subang (SB), and Ujung Kulon (UK), respectively. The hemolymph was collected from each individual and immediately preserved in absolute ethanol. Field experiments were approved by the Research Council of the Study Program from IPB University (letter number 1426/IT3.F3.2/KP.03.03.2019).
Figure 1: Sampling locations of Tachypleus gigas; There were eight, 14, 16, 13, 20, and 20 samples from Bintan Island (BT) = 8, Balikpapan (BP) = 14, Demak (DK) = 16, Madura (MD) = 13, Subang (SB) = 20 and Ujung Kulon (UK) = 20.
Genomic DNA extraction, amplification, and DNA sequencing
Genomic DNA was isolated from each hemolymph sample following a Genomic DNA Mini Kit (Geneaid, New Taipe, Taiwan) according to the manufacturer’s instructions. A fragment of the AT-rich region was amplified using a pair of primers, Hb-12S (5′-GTCTAACCGCGGTAGCTGGCAC-3′) and Hb-trna (5′GAGCCCAATAGCTTAAATTAGCTTA-3′), designed from the mitochondrial genome of the American horseshoe crab (Lavrov, Boore & Brown, 2000). A 25-µL PCR reaction was carried out with 12.5 µL MyTaq HS Red Mix (Meridian Bioscience, OH, United States), 9 µL ddH2O, 1.25 µL forward and reverse primer, and 1 µL DNA template. The entire reaction mixture was amplified using a peqSTAR thermal cycler (Peqlab, Erlangen, Germany), following Yang et al.’s (2007) amplification steps. The mixture underwent pre-denaturation at 95 °C for 3 mins, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 1 min, extension at 72 °C for 2 min, one cycle at 72 °C for 2 min, and 25 °C for 5 min. The PCR product was visualized using electrophoresis on a 1% agarose gel in TAE buffer with ethidium bromide at 100 V for 30 min. After electrophoresis, the gel was placed under UV light for band detection to determine the presence of a DNA fragment. The DNA sequencing was performed by 1st BASE DNA Sequencing Services, Selangor, Malaysia.
Data analysis
A total of 91 AT-rich region sequences were obtained, and MEGA X (Kumar et al., 2018) was used to generate multiple alignments of the edited sequences. Genetic diversity was measured using the number of haplotypes (Hn), haplotype diversity (Hd) and nucleotide diversity (π) using DNASp v6 (Rozas et al., 2017). The population structure was assessed using Wright’s fixation index (FST) and analysis of molecular variance (AMOVA). The significance level threshold (α), used to determine the pattern of differentiation between locations, was 0.05. The pairwise F-statistic (FST) was calculated as the genetic distance based on the population differences using DNASp v6 (Rozas et al., 2017). The haplotype network across populations was estimated using a median joining (MJ) network (Bandelt, Forster & Röhl, 1999) and was calculated using Network v 4.6.1.0 based on haplotype data. The haplotype composition across all study areas was illustrated in a map to show distribution and genetic connectivity patterns across the populations. Tajima’s D (1989) and Fu’s FS (1997) statistical tests were used to assess the population equilibrium using the Arlequin v.3.5 program (Excoffier & Lischer, 2010).
Results
Genetic diversity
We obtained a total of 91 AT-rich sequences of approximately 670 bp across all sampling locations including Java (UK, SB, DK, and MD), Sumatra, Bintan and Borneo (Balikpapan). In total, 43 variable nucleotide sites and 34 haplotypes were observed. The haplotypes consisted of both unique (found only in certain locations) and common haplotypes (Table 1). The genetic diversity of the coastal horseshoe crab varied across sampling sites (Table 2). The percentage of A+T composition at each location, which differed slightly, was approximately 81%.
| Nucleotide positions | n | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | |||||
| 2 | 3 | 8 | 3 | 7 | 7 | 6 | 6 | 6 | 6 | 7 | 8 | 1 | 4 | 4 | 4 | 5 | 6 | 7 | 7 | 7 | 8 | 0 | 1 | 1 | 1 | 3 | 3 | 4 | 6 | 7 | 7 | 9 | 9 | 0 | 6 | 6 | 6 | 8 | 2 | 4 | 7 | 8 | ||
| 5 | 2 | 3 | 5 | 4 | 8 | 0 | 1 | 6 | 9 | 4 | 2 | 3 | 2 | 3 | 4 | 9 | 6 | 2 | 3 | 4 | 6 | 1 | 3 | 4 | 5 | 0 | 7 | 6 | 7 | 2 | 7 | 1 | 2 | 2 | 3 | 6 | 7 | 9 | 0 | 7 | 2 | 5 | ||
| H1 | T | T | C | C | C | T | G | A | C | A | C | T | T | C | A | A | C | T | T | A | T | A | C | T | T | T | G | A | T | T | A | A | A | C | C | T | A | A | G | C | T | G | C | 7 |
| H2 | C | C | . | . | . | C | A | . | . | . | A | . | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | G | . | C | . | . | . | T | . | . | . | . | . | A | A | . | . | 1 |
| H3 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | 15 |
| H4 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | 1 |
| H5 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 12 |
| H6 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | . | . | . | . | . | . | 2 |
| H7 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | T | . | . | . | . | . | . | . | . | 3 |
| H8 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | A | . | . | 5 |
| H9 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | 6 |
| H10 | C | C | T | . | T | C | A | G | . | . | . | C | . | T | G | T | . | . | . | . | C | . | T | . | C | C | A | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | 1 |
| H11 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | T | C | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | A | . | . | 1 |
| H12 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | 1 |
| H13 | C | C | T | . | . | C | A | . | T | . | . | C | . | . | . | T | . | . | . | . | . | G | . | . | . | . | . | . | . | C | . | . | G | . | . | . | G | . | . | . | A | . | . | 1 |
| H14 | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 2 |
| H15 | C | C | . | . | T | C | A | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | A | . | . | 1 |
| H16 | . | . | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H17 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | A | . | . | 2 |
| H18 | . | C | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 9 |
| H19 | . | C | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | C | C | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H20 | . | C | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H21 | C | C | T | . | T | C | A | . | . | . | . | C | . | . | . | T | . | . | . | . | . | G | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | 1 |
| H22 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | A | . | . | 1 |
| H23 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | A | . | . | 3 |
| H24 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | A | . | 1 |
| H25 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | T | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | . | . | A | . | . | 1 |
| H26 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | 1 |
| H27 | . | C | . | . | . | . | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H28 | . | C | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | 1 |
| H29 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | . | . | . | . | . | . | . | A | . | . | 1 |
| H30 | C | C | . | . | . | C | A | . | . | . | . | . | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | A | . | . | 3 |
| H31 | . | C | . | T | . | . | . | . | . | . | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H32 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | G | C | . | . | . | . | . | . | . | . | C | G | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H33 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | T | . | . | G | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
| H34 | . | C | . | . | . | . | . | . | . | G | . | . | C | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | 1 |
Notes:
- n
-
number of observations of each haplotype
| Population | Code | A+T% | n | Nh | h | π |
|---|---|---|---|---|---|---|
| Bintan | BT | 81.597 | 8 | 6 | 0.892 | 0.006 |
| Balikpapan | BP | 81.473 | 14 | 10 | 0.945 | 0.009 |
| Demak | DK | 81.568 | 16 | 6 | 0.783 | 0.004 |
| Madura | MD | 81.412 | 13 | 8 | 0.910 | 0.006 |
| Subang | SB | 81.548 | 20 | 11 | 0.926 | 0.005 |
| Ujung Kulon | UK | 81.434 | 20 | 12 | 0.942 | 0.005 |
| Total | 91 | 0.935 | 0,0064 | |||
Notes:
- n
-
number of samples
- Nh
-
number of haplotype
- h
-
haplotype diversity
- π
-
nucleotide diversity
At a glance, the obtained haplotype diversity was high, ranging from h = 0.783 to 0.945 with a mean gene diversity per population h = 0.935. Conversely, the nucleotide diversity was relatively low in all locations, ranging from π = 0.004 to 0.009. The overall diversity was similar across populations. DK had the lowest haplotype and nucleotide diversity (h = 0.783, π = 0.004). BP had the highest haplotype and nucleotide diversity (h = 0.945 π = 0.009), followed by UK (h = 0.942, π = 0.005), SB (h =0.926, π = 0.005), MD (h =0.910, π = 0.006), and BT (h = 0.892, π = 0.006) (Table 2).
Population structure
Pairwise FST values ranged from 0 to 0.13 across the populations (Table 3). Generally, the FST value among locations was not significantly different from zero (p > 0.05) with the exception of UK-MD and UK-SB, indicating the restricted gene flow among these populations. Populations with higher pairwise FST values included BT-MD (p > 0.05), BT-SB (p > 0.05), UK-MD (p < 0.05), and UK-SB (p < 0.05). The pairwise FSTvalues of UK-BT, DB-DK, and SB-MD were effectively zero. Our AMOVA results showed that the majority of variation was found within (95.23%) rather than among (4.77%) populations (Table 4).
| BT | BP | DK | MD | SB | UK | |
|---|---|---|---|---|---|---|
| BT | – | |||||
| BP | 0.05 | – | ||||
| DK | 0.08 | 0.00 | – | |||
| MD | 0.13 | 0.00 | 0.00 | – | ||
| SB | 0.11 | 0.01 | 0.00 | 0.00 | – | |
| UK | 0.00 | 0.08 | 0.09 | 0.10* | 0.10* | – |
Notes:
FST value significantly different (p < 0.05)*.
- BT
-
Bintan
- BP
-
Balikpapan
- DK
-
Demak
- MD
-
Madura
- SB
-
Subang
- UK
-
Ujung Kulon
| Source of variation | d.f | Percentage of variation | FST | p-values |
|---|---|---|---|---|
| Among populations | 5 | 4.77 | 0.04 | 0.006 |
| Within populations | 85 | 95.23 | ||
| Total | 90 |
Population connectivity
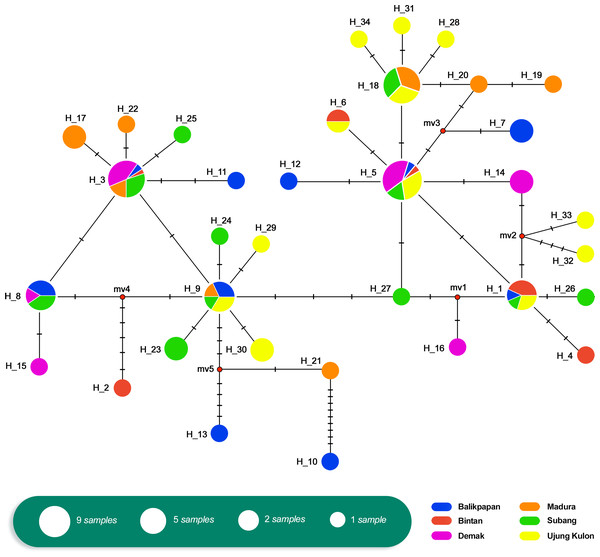
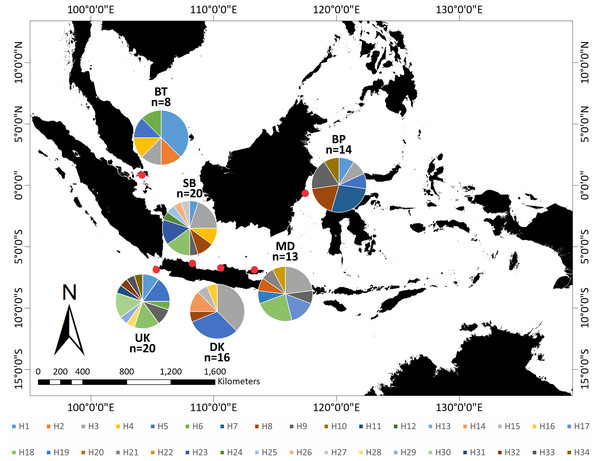
The relationship of the 34 haplotypes was illustrated using a median-joining network (Fig. 2). The haplotype network showed that there were shared haplotypes (H1, H3, H5, H6, H8, H9, and H18) across the geographic sites. H3 was the most common, and was identified in all populations except UK and including 15 individuals. H5 was found in 12 individuals from the BT, BP, DK, SB, and UK populations. However, specific haplotypes were only found in certain locations. The UK population had the highest number of specific haplotypes (seven). Meanwhile, BT had the lowest number of haplotypes (two) (Fig. 3).
Figure 2: Haplotype network of Tachypleus gigas (n = 91) population in six locations around Indonesia, constructed with Median-Joining method.
Figure 3: Distribution of 34 haplotypes of Tachypleus gigas population from six locations in around Indonesia.
We assessed historical demography based on mtDNA AT-rich region haplotype frequencies. There were shared haplotypes in all locations (Fig. 2). Furthermore, the Tajima’s D test values (Table 5) were negative across all populations, with the exception of DK, MD, and SB. They showed no significant p-values, indicating that there was no evidence of selection. Similarly, the Fu’s F s test results (Table 5) were negative (except in DK), with no significant p-values across all six populations. This indicated an excess number of haplotypes, as expected due to a recent population expansion.
Discussion
In this study, there was high haplotype diversity in six coastal horseshoe crab populations in the northern Java Sea, Bintan, and Balikpapan waters of Indonesia. There was also a high number of polymorphic sites (43, with 34 defined haplotypes) in Indonesian coastal horseshoe crab populations. The mean haplotype diversity (h = 0.935) was quite high, while nucleotide diversity (π = 0.006) was low across all populations. Similarly high haplotype diversity values were reported in T. gigas (h = 0.797 ± 0.129 and π = 0.058 ± 0.001; Rozihan & Ismail, 2011) in Malaysia and tri-spined horseshoe crab (T. tridentatus) in Taiwan (h = 0.626 ± 0.075 and π = 0.003 ± 0.005; Yang et al., 2007).
Previous studies reported generally high genetic diversity in coastal horseshoe crab (Rozihan & Ismail, 2011; Aini et al., 2020). Our results showed not only high genetic diversity, but also low nucleotide diversity. The high number of haplotypes indicates that these populations were large enough to maintain a high level of genetic diversity. These small differences are the signature of rapid demographic expansion from a small effective population size (Avise, 2000). Nucleotide diversity is a sensitive index when analyzing population genetic diversity (Nei & Li, 1979), and is influenced by life-history characteristics, environmental heterogeneity, population size (Nei, 1987; Avise, 2000), fishing pressure (Madduppa, Timm & Kochzius, 2018), level of larval transport, and degree exchange with other populations (Timm et al., 2017). The rate of mitochondrial evolution and historical factors play an important role in determining genetic variability patterns (Grant, Spies & Canino, 2006; Xiao et al., 2009; Yamaguchi, Nakajima & Taniguchi, 2010).
| Population | Tajima’s D | Fu’s FS |
|---|---|---|
| Bintan | −0.646ns | −0.608ns |
| Balikpapan | 0.601ns | 0.847ns |
| Demak | 0.325ns | −2.941ns |
| Madura | −0.875ns | −1.532ns |
| Subang | 0.166ns | −0.891ns |
| Ujung Kulon | −0.318ns | −3.865ns |
Notes:
- ns
-
not significant
We detected low differentiation across populations (insignificant FST values between 0 and 0.13), with exceptions between populations UK-MD and UK-SB. This result indicated that there was little subdivision across populations. Several studies suggested restricted dispersal abilities for horseshoe crabs regarding short-term tagging. However, some others explained that this crab has a wide dispersal abilities based on long-term studies. Individual distances up to 30 km have been observed in Malaysian crabs (Mohamad et al., 2019), while the movement abilities of tri-spined horseshoe crab did not exceed 150 km (Yang et al., 2007). Similarly, the American horseshoe crab in the Great Bay Estuary (USA) has a maximum mean annual linear distance ranging between 4.5 km and 9.2 km (Schaller, Chabot & Watson, 2010). Studies by Swan (2005) over multiple years found that Limulus moved from 104 to 265 km from their release sites. Ecological observations showed that their hatched larvae swim freely for approximately 6 days and then settle in the bottom of shallow waters around their natal beaches (Shuster Jr, 1982). However, larvae have a strong tendency to concentrate in inshore rather than offshore waters (100–200 km) (Botton & Loveland, 2003), suggesting a limited ability for long-range dispersal between estuaries. Additionally, low FST levels reflect inter-population movement over mutigenerational intervals that short-term tagging studies cannot document. Long-term tagging studies have found that horseshoe crabs can move from >5–500 predominated km 5–30 km (Beekey & Mattei, 2015), and up to 767 km over their long lifetimes (E. Hallerman, 2020, personal communication). Long-term tagging study similar study by Rozihan & Ismail (2011) reported that the crab’s FST value along the west coast of peninsular Malaysia ranges from 0.111–0.557, indicating moderate to high genetic differentiation (Wright, 1978; Hartl & Clark, 1997). Other reports in the area used microsatellite markers to find a FST value between 0.144 and 0.846.
There were only seven shared haplotypes among the 34 total haplotypes observed among all 91 samples. The median-joining network analysis indicated past population expansions with shared haplotypes among localities. Overall, relationship patterns at the mtDNA level showed little geographical structure. The haplotype network revealed recent demographic processes, but the small sample sizes also limited the possibility of observing the intermediate haplotypes inferred to exist in the network. Moreover, results of Tajima’s D and Fu’s F s tests indicated the occurrence of population expansion. Common haplotypes shared between localities also can be explained by the historical biogeography in this Southeast Asian region known as the Sunda Shelves, which includes Java, Sumatera, and Borneo. Historically, Sundaland experienced both dewatering and inundation during the Pleistocene period. Haplotype sharing in this study is attributed to breeding migration and dispersal of pelagic larvae, as well as the sharing of common ancestors (Frankham, 1996). The occurrence of many geographic site-specific haplotypes can be explained by the small sample size and perhaps historical isolation during the Last Glacial Maximum. Many species became isolated in refugia, and genetic differentiation and divergence occurred due to the retreat and dispersal of glacial ice sheets (Hewitt, 2000).
A proactive management approach regarding the Asian coastal horseshoe crab (T. gigas) in Indonesia should consider population genetics. High haplotype diversity that occurs with low nucleotide diversity has been associated with population growth or expansion after a period of low effective population growth (Grant & Bowen, 1998). Our findings indicate that T. gigas in Indonesia have low genetic differentiation but high population connectivity and expansion. Therefore, our results suggest that there is a single stock of Indonesia coastal horseshoe crab. The best conservation strategy would be one that combines both local and regional management. To expand our knowledge base, an advanced population genetic analysis based on male and female horseshoe crabs and the nuclear genome (e.g., microsatellites or SNPs) should be conducted. This should also include expanding the scope of geographic sampling around Indonesia.
Conclusion
High genetic diversity and low levels of differentiation across coastal horseshoe crab (T. gigas) populations in Indonesia indicated a single stock with high connectivity. A locally and regionally based conservation management method is suggested as a precautionary approach to conserving the Indonesian coastal horseshoe crab.