Abstract
Tris(8-hydroxyquinoline)aluminum (Alq3) has been widely applied as a good electron-injecting layer (EIL) in organic light-emitting diodes. High-sensitivity photoemission measurement revealed a clear photoemission by visible light, although its ionization energy is 5.7 eV. This unusual photoemission is ascribed to Alq3 anions captured by positive polarization charges. The observed electron detachment energy of the anion was about 1 eV larger than the electron affinity reported by inverse photoemission. This difference suggests that the injected electron in the Alq3 layer is energetically relaxed, leading to the reduction in injection barrier. This nature is one of the reasons why Alq3 worked well as the EIL.
Export citation and abstract BibTeX RIS
Since the epoch-making improvement of organic light-emitting diodes (OLEDs) by Tang and VanSlyke in 1987,1) tris(8-hydroxyquinolate)aluminum (Alq3) has been widely applied to OLEDs. One of the factors for Tang's breakthrough is the use of Alq3 as the emission and electron-injecting material. The ability of this material to inject electrons is ascribed to the relatively large electron affinity of 1.96 eV determined by inverse photoemission.2) However, this value is not comparable to the work function (Φm) of a typical cathode metal such as Al (Φm = 4.3 eV) or Ca (Φm = 3.0 eV).3) In addition to electron affinity, orientation polarization in an amorphous Alq3 film was also proposed as the reason for the electron injection nature as described below.
For Alq3 films, weak orientation polarization was proposed by Ito et al. in 2002 from the surface potential measurement.4) They discovered the evolution of the surface potential proportional to the film thickness at a rate of about 50 mV/nm and named this peculiar phenomenon as "giant surface potential (GSP)". This potential cannot be neglected because the practical thickness of OLEDs can lead to a potential drop of several volts, which is compatible with the driving voltage. A vacuum evaporation process can induce this orientation polarization without any poling, in contrast to ferroelectric polymer materials. Subsequent studies revealed that this phenomenon is not specific to Alq3 but is quite common. Positive GSP, where the surface (substrate) side is charged positively (negatively), is observed in most cases reported previously.5,6,7) Because of these positive and negative fixed charges on both ends of the polarized layer, interface charges are formed in OLEDs.5,8) On the other hand, Isoshima et al. reported that a derivative of Alq3, tris(7-propyl-8-hydroxyquinolinato)aluminum [Al(7-Prq)3], shows the reversed polarity of GSP (negative GSP).9) Noguchi et al. performed displacement current measurement (DCM)10) of Alq3- and Al(7-Prq)3-based OLEDs to examine the impact of the polarity of GSP on the device performance. They suggested that the contact resistance of the Alq3/cathode interface is lower than that at the Al(7-Prq)3/cathode interface, indicating the importance of GSP polarity.11) The lower resistance of the Alq3-based device was speculated to be due to the attractive interaction between the injected electron and the positive polarization charge near the cathode interface, leading to the reduction in electron injection barrier. To clarify this mechanism, direct examination based on the electronic structure is highly desired.
Our study aims to directly demonstrate such a stabilization effect of electrons on the Alq3 film surface through the observation of the electronic structure using high-sensitivity photoelectron spectroscopy (HS-PES)12,13) and photoelectron yield spectroscopy (HS-PYS).12,14,15) Although PES usually uses high-energy photons as an excitation light source, we observed an unusual low-energy photoemission from Alq3 films using visible light and ascribed it to one-photon photoemission from the anion state of Alq3 (this carrier state is often called "electron" in the organic device field but should be considered as "radical anion") captured on the film surface because of the polarization charge. The observed electron detachment energy of the anion was about 1 eV larger than the electron affinity of Alq3 determined by inverse photoemission2) because the anion is relaxed by Coulomb interaction with a positive polarization charge. The small electron injection barrier height, which was suggested in Ref. 11, can be ascribed to this energy relaxation effect. This is why Alq3 shows good electron injection nature.
HS-PES and HS-PYS experiments were performed using a homemade photoemission measurement system.12,14,15) Sublime-grade Alq3 and Al(7-Prq)3, whose chemical structures are shown in Fig. 1(a), were used as received and thermally evaporated onto indium tin oxide (ITO) substrate under dark condition. A thickness of 30 nm was selected to exclude the photoemission signal from the substrate; the film thickness is larger than the expected mean free path (<10 nm) of low-energy electrons (<5 eV).16) In the HS-PES measurement, monochromatized photons from a D2 lamp and a Xe lamp were used as an excitation light source. A specially designed monochromator effectively excludes stray photons to suppress the background level in PES spectra leading to high sensitivity. The incident angle was 55° from the surface normal, and a hemispherical analyzer was fixed at the normal emission angle. The very small photocurrent (<1 pA) suppressed any sample charging. HS-PYS was conducted with the same system using the Xe lamp for the same sample with HS-PES. The photoelectron was detected by a channeltron. No spectral shift due to contamination and/or damage was observed.
Fig. 1. Upper and lower panels are for Alq3 and Al(7-Prq)3, respectively. (a) Chemical structures of the sample molecules. (b) Energy position of vacuum level (squares) and HOMO onset (circles) during PES measurement (hν = 7.7 eV) as a function of measurement time. (c) Schematics of the decay of the positive (Alq3) and negative [Al(7-Prq)3] GSPs by photocarrier generation. (d) Accumulation of the negative (Alq3) and positive [Al(7-prq)3] carriers as compensation charge.
Download figure:
Standard image High-resolution imageFigure 1(b) shows the variations of the highest occupied molecular orbital (HOMO) onset and vacuum level (VL) in the PES (hν = 7.7 eV) (typical spectra are shown in supplementary data, Fig. S1 in the online supplementary data at http://stacks.iop.org/APEX/9/021601/mmedia) of Alq3 and Al(7-Prq)3 films as a function of measurement time. In the case of the Alq3 film [upper panel of Fig. 1(b)], the initial work function (3.21 eV) was smaller than that of ITO (4.16 eV), indicating the formation of positive GSP. During the PES measurement, the VL and HOMO of Alq3 shifted upward in parallel, indicating the decay of the positive GSP. Photoemission processes create cations (photo-holes) and primary/secondary electrons, parts of which are attached to the molecules to form anions. Anions thus formed are expected to drift to the surface to compensate for positive polarization charges as shown in the upper panels of Figs. 1(c) and 1(d).
As in the lower panel of Fig. 1(b), the initial work function of the Al(7-Prq)3 film (5.27 eV) was larger than that of ITO (5.15 eV), suggesting the formation of negative GSP. In contrast to Alq3, a downward spectral shift was observed during PES measurement, indicating the compensation of the negative GSP by cations as shown in the lower panels of Figs. 1(c) and 1(d). Thus, we prepared two samples with positive and negative compensation charges; anions and cations are expected to accumulate on the surfaces of the Alq3 and Al (7-Prq)3 films, respectively.
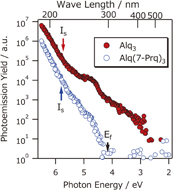
HS-PYS spectra of the two samples are shown in Fig. 2. From the linear extrapolation of the cubic root of yield obtained by normal sensitivity measurement [shown in Fig. S1(a) and S1(b) in the online supplementary data at http://stacks.iop.org/APEX/9/021601/mmedia], the ionization energies (I) of Alq3 and Al(7-Prq)3 are determined, as shown by the arrows. The observed I of 5.73 eV for Alq3 is consistent with literature values of 5.7 eV11) and 5.8 eV.17) The I of Al(7-prq)3 is 5.80 eV, which is very similar to that of Alq3. This is because the propyl group does not effectively modify the π-electron system in Alq3. This similarity was also confirmed by molecular orbital calculation using Gaussian 09.18) Both spectra have a tail structure in the lower energy region below the ionization threshold. The tail structure of Al(7-Prq)3 extends close to the Fermi level of the ITO substrate because of the gap state,13,15) while that of Alq3 does to smaller energy regions up to 2.8 eV. This value is much smaller than the ionization energy (5.7 eV). It should be noted that photoemission is usually induced by deep UV or higher energy photons.
Fig. 2. HS-PYS spectra of Alq3 and Al(7-Prq)3 films. Only Alq3 shows a long tail up to the visible light region.
Download figure:
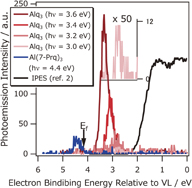
Standard image High-resolution imageTo clarify the origin of this anomalous photoemission event, HS-PES measurement for low hν was performed, as shown in Fig. 3. For the Al(7-prq)3 (hν = 4.4 eV) spectrum, photoemission was detected and the onset was 4.2 eV, similar to the HS-PYS result. For lower photon energies, no signal was detected. In contrast, a clear photoemission was detected for Alq3 over the hν = 3.0–3.6 eV range. Even for hν = 3.0 eV, which is 2.7 eV smaller than the I of Alq3, a clear photoemission was detected. The emission threshold for the spectra was approximately 2.5 eV, similar to the PYS onset. Furthermore, the observed spectra of the Alq3 film are located above the Fermi level of the ITO substrate, while those of the Al(7-prq)3 film are below the Fermi level. Such a shallow photoemission peak compared with the Fermi level is often observed in two-photon photoemission using laser light. However, from the photon energy and intensity dependences, the two-photon process is clearly excluded (see the online supplementary data at http://stacks.iop.org/APEX/9/021601/mmedia). Thus, we conclude that the occupied shallow state actually exists above the Fermi level.
Fig. 3. Series of HS-PES spectra of Alq3 and Al(7-prq)3 films for hν = 3.0, 3.2, 3.4, 3.6, and 4.4 eV. The inset shows the magnified spectrum of Alq3 for hν = 3.0 eV. Even for hν = 3.0 eV, a photoemission was detected. However, the threshold of Al(7-prq)3 is 4.6 eV. The result of IPES from Ref. 20 is also displayed as the black spectrum.
Download figure:
Standard image High-resolution imageFrom the above results, the observed shallow state is ascribed to the Alq3 anion. As described above, the anions (cations) are expected to accumulate on the surface of the Alq3 [Al(7-Prq)3] film during the PES measurement. The ionization energy of the cation is similar or slightly larger than that of the neutral molecule because the positive charge of the cation stabilizes the electronic energy of the HOMO level. On the other hand, the electron detachment energy of the anion (the smallest energy to remove an electron from an anion) approximately corresponds to the electron affinity of the neutral molecule, if we ignore the intra- and intermolecular relaxation effects. Thus, the observed low-energy photoemission can be considered to originate from the Alq3 anion captured by positive polarization charge. On the other hand, photoemission from the Al(7-Prq)3 cation cannot be induced by visible light because a photon with an energy higher than the ionization energy (5.7 eV) is necessary. Hence, these results demonstrate that we succeeded in observing the Alq3 anion state using negative ion photoemission spectroscopy (NI-PES). To the best of our knowledge, this corresponds to the first demonstration of negative ion photoelectron spectroscopy of organic solid-state systems, except chemically doped systems.19) NI-PES is a powerful tool that can be used to directly investigate the electronic structure of the anion state; the electron affinity (A) and unoccupied density of states of organic semiconductor materials can be observed without any damage.
Next, we compare the results between NI-PES and IPES. Figure 3 shows the IPES spectrum of Alq32) and the NI-PES spectrum observed in this work. The shallower side of the secondary electron cutoff peak of the NI-PES spectrum for hν = 3.6 eV is regarded as the expected density of states (DOS) of the Alq3 anion. In general, the PES spectrum includes primary and secondary electrons. The former approximately reflects the DOS of a sample; however, the latter induces a background peak in the low-kinetic-energy region. In this study, the excitation of secondary electrons is negligible because of the low excitation energy; 3–4 eV is insufficient to induce an energy-loss process to create many secondary electrons. The spectra show a monotonic increase from onset to secondary electron cutoff, and a monotonically changing DOS is expected with no peak. The threshold energy of the NI-PES is approximately similar to that of the IPES (1.96 eV below VL). The IPES spectrum, which roughly reflects the unoccupied density of states (UDOS), extends upwards, while the NI-PES spectrum (DOS of the anion) extends downwards. The DOS of the Alq3 anion is located below the UDOS obtained by IPES. To clarify this point, the essential difference between the two methods will be discussed next.
The excitation processes for a molecule (M) in IPES and NI-PES can be described as
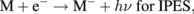
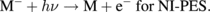
The energy balances for these processes are
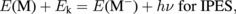
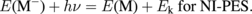
Here, Ek is the electron kinetic energy, and E(M) and E(M−) are the total energies of the neutral molecule and anion, respectively. Thus, the electron affinity obtained from IPES (AIPES) corresponds to the electron detachment energy obtained from NI-PES (DNI-PES) given as
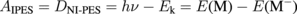
under frozen orbital approximation. Actually, AIPES and DNI-PES are different in terms of intra- and intermolecular relaxation energy. To estimate intramolecular relaxation, AIPES and DNI-PES are calculated for isolated molecules by the ΔSCF method (Gaussian 09,18) B3LYP). The obtained intramolecular relaxation energy is 0.25 eV (= DNI-PES − AIPES = 0.80 eV − 0.55 eV). As intermolecular relaxation, we should consider the relaxation of the anion by the surrounding molecules, the so-called polaronic effect. The main relaxation process in PES and IPES is electronic polarization, where the ion in the final state is relaxed by the polarization of the electron cloud of the surrounding molecules. This is a very fast process that occurs on a time scale of 10−16 s. Hence, AIPES reflects the relaxation effect because of the electronic polarization. In the case of NI-PES, the anion is not in the transient final state but in a steady initial state. The anion state has sufficient time to be fully relaxed not only by the fast electronic polarization but also by the rather slower processes such as vibronic polarization (10−15–10−14 s), lattice polarization (10−14–10−13 s), and intermolecular geometrical deformation (quite slow).20,21)
In principle, this difference suggests that the NI-PES is expected to probe the electronic structure of fully relaxed carriers in organic semiconductors more realistically; hopping transport in OLED gives enough time for carrier relaxation. However, the above slow relaxation process results in a small relaxation energy of only 0.1–0.2 eV at maximum.21) To explain the observed difference, another type of relaxation should be considered.
In our model, the anion of Alq3 is captured by the fixed positive polarization charge on the surface. Therefore, Alq3 anions are relaxed by both the polaronic effect and the ion-charge Coulomb electrostatic interaction. The Coulomb interaction between the anion and the polarization charge is a possible reason for the difference. At present, the spatial distribution of the captured anions is unclear, and the Coulomb interaction cannot be quantitatively estimated. To obtain an estimate, we discuss the difference between the anion state in the charge-transfer exciton and the free anion state. In the former case, the anion is stabilized by Coulomb interaction with the adjacent cation regarded as a charge–charge Coulomb interaction system. This situation is similar to our Coulomb interaction system where the anion is stabilized by a polarization charge. The anion state should be stabilized by a binding energy with a counter charge [EB(relax)]. EB(relax) can be estimated from the difference between the transfer gap (3.64 eV from IPES and PES2,17)) and the optical gap (2.75 eV11)) of Alq3. Thus, an approximately 0.9 eV (= 3.64–2.75 eV) downward shift of the DOS is possible when the anion is stabilized by a counter charge. This argument can explain the observed difference of about 1 eV.
Another point to differentiate NI-PES from IPES is the line shape of the DOS. The UDOS obtained by IPES shows a peak structure because of LUMO at ∼1 eV from LUMO onset; however, the DOS obtained by NI-PES shows a monotonic increase with no peak in the range of at least 1 eV from NI-PES onset. This can be ascribed to the spatial distribution of the captured anions. The different position of anions relative to the polarization charge gives a different relaxation energy. The onset region of NI-PES spectra is due to the anion that is located in the bulk region far from the polarization charge. The anion state energy approaches the relaxed anion state energy only through the electric polarization obtained by IPES.
Alq3 polarization is suggested to be related to the carrier injection nature. Noguchi et al. reported the difference in contact resistance between Alq3- and Al(7-prq)3-based devices.11) At the cathode interface, the former shows a better contact because of the positive polarization charges that assist the electron injection from the cathode. The observation using NI-PES in this work clearly suggested that (i) the positive polarization charge due to GSP captures anions and energetically stabilizes them by the order of electron volt (to lower the injection barrier), (ii) the energy of the anion state near the surface region has a large distribution, and (iii) the energy gap evaluated by PES and IPES may be overestimated for practical OLED systems.
In summary, using HS-PES and HS-PYS, we directly demonstrated that the anion state on the surface of polarized Alq3 film is energetically stabilized to reduce the effective injection barrier. This is why Alq3 shows good electron injection nature and how orientation polarization improves the OLED performance. Since such a weak orientation polarization is a common phenomenon among organic amorphous materials, we have to pay attention to this to discuss the device performance. This can be regarded as a type of self-field carrier doping as indicated below. In organic field-effect transistors, carriers accumulate in the channel because of the field effect caused by gate bias. In the case of films with GSP, carriers are spontaneously accumulated at both ends of the layer by the internal self-field due to the polarization charge. The energetic relaxation of anions by the polarization charge can be utilized to control the performance of various devices. Our method of investigating the anion state by photoemission can be improved to extend its use as a tool to probe the unoccupied state of organic materials without sample damage; very recently, we have obtained a similar low-energy photoemission for other OLED materials.22) Thus, it can be a powerful method of probing the carrier state in realistic situations in practical devices.
Acknowledgments
We would like to thank Dr. S. Machida of Panasonic Corporation for his kind cooperation in our experiment. We would also like to thank Nippon Steel & Sumitomo Metal Corporation for providing Alq3 samples. This research was supported by JSAP through the "Funding Program for World-leading Innovative R&D on Science and Technology (FIRST Program)" initiated by the Council for Science and Technology Policy and KAKENHI (No. 25288114), and the Global-COE project of Chiba University (Advanced School for Organic Electronics).