Do we need a revised staging system for malignant pleural mesothelioma? Analysis of the IASLC database
Introduction: A number of staging systems have been proposed for malignant pleural mesothelioma (MPM)
in the past, but few have utilized a TNM (tumor, node, metastasis) system. The International Association for
the Study of Lung Cancer (IASLC) and the International Mesothelioma Interest Group (IMIG) previously
developed a TNM-staging system which has been accepted by the International Union Against Cancer
(UICC) and the American Joint Commission on Cancer (AJCC). The present study examines this staging
system by analysing the updated IASLC database for patients with MPM.
Methods: De-identified data from participating centres dated from 1995 to 2009 were submitted to the
IASLC Statistical Center. Surgical procedures included those with a curative or palliative intent. Survival
was measured from the date of pathologic diagnosis to the most recent contact or death. Endpoints included
overall survival and analysis of potential prognostic factors.
Results: Data was available for 3,101 patients from 15 centers, mostly from North America and Europe.
After a median follow-up of 15 months, a number of clinicopathological and treatment-related prognostic
factors were found to significantly influence overall survival. These included overall tumor stage based on the
proposed TNM staging system, T category, N category, tumor histology, gender, age, and type of operation.
Conclusions: The IASLC database represents the largest, multicenter and international database on MPM
to date. Analyses demonstrate that the proposed TNM staging system effectively distinguishes the T and N
categories, but also highlight areas for potential revision in the future.
Key words: pleural mesothelioma; extrapleural pneumonectomy; pleurectomy; decortication; trimodality therapy; multi-institutional database; survival; staging
Introduction
Despite recent advances in treatment for malignant pleural mesothelioma (MPM), long-term survivors of this difficult malignancy are rare. A well validated staging system is essential to evaluating new therapies for MPM. Until the mid-1990s no such staging system existed. At least 6 staging paradigms had been proposed, none evidence-based, and few utilizing a TNM (tumor, node, metastasis) system. In 1994, at a workshop sponsored by the International Association for the Study of Lung Cancer (IASLC) and the International Mesothelioma Interest Group (IMIG), MPM investigators analyzed existing surgical databases to develop a TNM-based staging system (1). This proposed staging system was accepted by the UICC (International Union Against Cancer) and the AJCC (American Joint Commission on Cancer) as the international MPM staging system for the 6th and 7th editions of their staging manuals (2,3). The IMIG staging system has since been widely used but questions about its validity persist because it is derived from analyses of small retrospective surgical series, can be difficult to apply to clinical staging and utilizes descriptors for lymph node involvement that may not be relevant to MPM. Therefore, in collaboration with IMIG, the IASLC decided to update the staging system for MPM by developing a large international database, an effort modeled on the revisions that the IASLC proposed for lung cancer staging for the 7th editions of the UICC and AJCC manuals (4). Initial analyses of the international IASLC/IMIG database for MPM have been reported and identified areas in which the current staging system warrants modification. This article succinctly reviews information from analyses of the IASLC database presented in more detail in a recent publication in the Journal of Thoracic Oncology (5).
Methods
Participation in the database was solicited from investigators active in the IASLC and/or IMIG. Initial analyses focused on MPM patients who had surgery as part of their care and therefore, presumably had earlier stage disease. Participating investigators submitted de-identified data from existing registries to the IASLC Statistical Center, Cancer Research and Biostatistics (CRAB) in Seattle, WA., USA. Common data elements were established after review of each institutional database at CRAB. The time frame chosen for data was from 1995 to 2009, considered a contemporary period for providing relevant staging information.
Surgical procedures were classified as either palliative or curative intent operations. The former included exploration without resection and partial pleurectomy while the latter included extrapleural pneumonectomy (EPP), pleurectomy/decortication for resection of all gross tumor (P/D) and P/D combined with anatomical lung resection other than pneumonectomy. Because of the diverse nature of the individual databases, details of chemotherapy and radiation were not available and were thus recorded only as modalities given or not given.
Survival was measured from date of pathologic diagnosis to the date of last contact or death due to any cause. Median survival was estimated using the Kaplan-Meier regression method. Prognostic groups were assessed by Cox regression analysis of survival. Significance values from pair-wise comparisons reflect the Wald test; those from joint model effects (e.g., comparing the full model to the null model) reflect the likelihood ratio test.
All covariates in regression analyses were modeled categorically using indicator variables, and the threshold for statistical significance was set at P=0.05. Covariates which met the criteria for statistical significance by univariate analysis were further evaluated for inclusion in multivariable regression models, using a stepwise algorithm with backward selection. Such models were considered “exploratory” in nature when 33% or more of the sample had to be excluded from analysis due to missing data for any of the covariates being explored.
Results
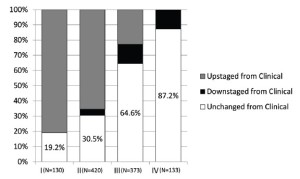
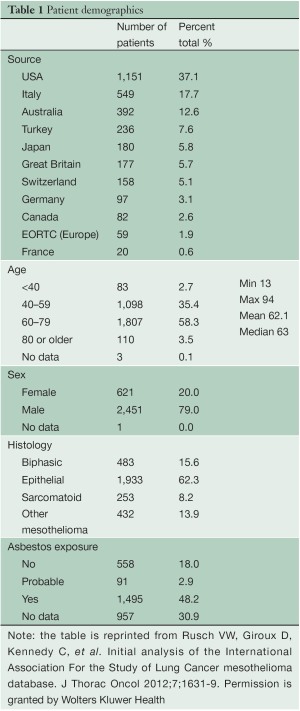
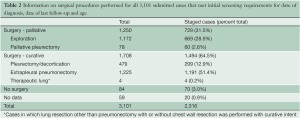
Data were submitted on 3,101 patients from 15 centers on 4 continents with 82.6% of the patients emanating from North America and Europe (Table 1). Patients were predominantly male with a median age of 63 years. Asbestos exposure was recorded in nearly half of the patients but data on this were lacking in 30.9% of cases. Epithelioid histology was the most common MPM subtype reported (60% of cases). Both clinical and pathological staging data were not available on all patients and thus clinical (cTNM) and pathological (pTNM) staging information were combined in 2,316 patients to provide “best” staging (bTNM) in accordance with AJCC and UICC guidelines. The majority of patients (64.5%) had curative intent procedures with approximately half undergoing EPP (Table 2). Upstaging based on final pTNM occurred in up to 80% of patients deemed to have clinical stages I or II disease but only in 22.8% of clinical Stage III tumors and not at all in Stage IV disease (Figure 1).
Full table
Full table
The median length of follow-up for all patients was 15 months. Survival by cTNM, pTNM and bTNM stage for all patients undergoing any type of surgical procedure is shown in Figure 2. Overall survival by bTNM for the patients undergoing surgery with curative intent demonstrates more obvious differences in median survival across all 4 tumor stages (Figure 3).
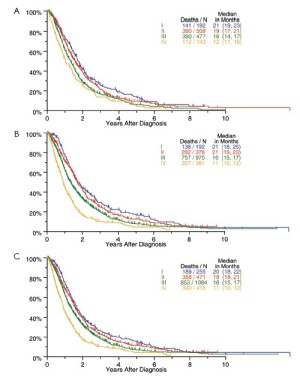
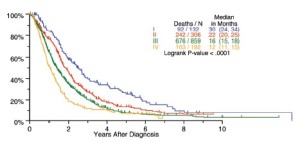
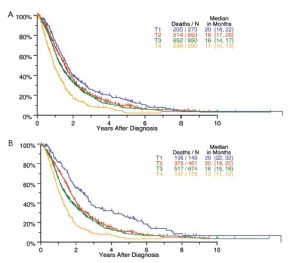
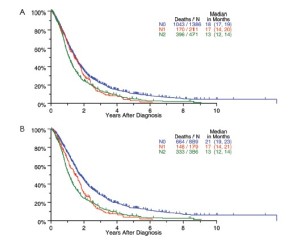
Survival by tumor T categories for all patients having nonmetastatic disease managed by any type of surgical procedure and for those undergoing operations with curative intent is shown in Figure 4. Separation is seen among the median survivals for all 4 T categories but this is least apparent between T2 and T3. Survivals by tumor N categories for patients undergoing any type of surgical procedure and for those having curative intent operations are shown in Figure 5. Differences are seen for N0 versus N1 versus N2 but with the predominant difference being between N0 and N1/N2.
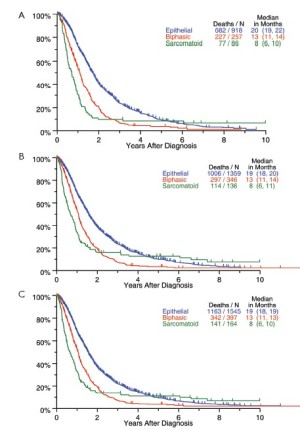
The relationship between histological subtype and survival for all patients undergoing surgery is shown in Figure 6. Pronounced differences in outcome were seen, with epithelioid histology being associated with the best outcome and sarcomatoid, the worst.
Survival was significantly different according to whether the surgical procedure was performed with curative versus palliative intent (median survival 18 versus 12 months,P<0.0001). Prognostic groups defined by the type of curative intent procedure performed (EPP versus P/D) were examined in relationship to tumor stage. Stage I tumors resected by EPP were associated with a median survival of 40 months while those managed by P/D had a median survival of 23 months. No differences in survival between EPP and P/D were identified in patients with higher stage disease.
Among the patients undergoing curative intent operations, 1,162 received additional treatment, either chemotherapy or radiation or both. Relative to the 207 patients in this group who were managed with surgical resection alone, the patients receiving multimodality treatment had a significantly better outcome with median survivals of 20 versus 11 months (P<0.0001).
Several multivariable analyses (Tables 3,4) were performed for patients undergoing any type of surgical procedure. Overall tumor stage (P<0.0001), T category (P<0.0001), N category (P<0.0001), tumor histology (P<0.0001), patient gender (P=0.0002) and age (P=0.0025), and type of operation (curative versus palliative, P<0.0001) had a statistically significant impact on survival. Likewise, pairwise comparisons of adjacent stage groups, T, and N categories yielded statistically significant differences in survival, with the exception of stages I vs. II, T1 vs. T2, and N1 vs. N2.
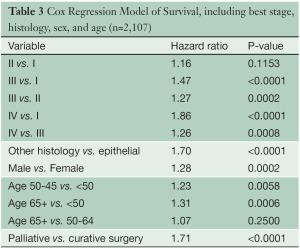
Full table
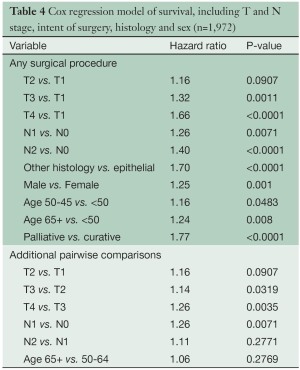
Full table
Discussion
Recent improvements in surgical treatment, chemotherapy and radiotherapy have led to an increasing use of multimodality therapy and to more clinical trials in MPM (6-12). An accurate staging system is essential in assessing the benefit of new therapies for this difficult disease. The current staging system and reports suggesting possible revisions are based on small, retrospective surgical series (13-19). Though retrospective, this IASLC database is the largest multicenter and international database in MPM to date.
Analyses of the IASLC database show that the current staging system distinguishes among T and N categories and overall stages but also highlight areas for potential revision. Differences in survival among T categories and overall stage classifications are most apparent among patients undergoing resection with curative intent. Unlike many other cancers where the size and location of the primary tumor can be reproducibly measured, the extent of the tumor in MPM is not easily measured. The current T descriptors are qualitative and most applicable to surgical and pathological staging. In the future, volumetric tumor measurement on computed tomography (CT) could replace the current T descriptors (20-26). However, this approach requires further study. For current purposes, revision of the descriptors for early stage disease (T1-3) is needed based on information regarding the anatomical extent of disease. Few participating institutions were able to provide information about the precise anatomical extent of tumor leading to the assignment of T categories. This information will be necessary to recommend definitive revision of T categories.
The application of lung cancer N categories to MPM in the original IMIG staging system was empiric because no data were available at the time to suggest alternative options. The grouping of both N1 and N2 disease into stage III disease was also empiric because all that was known at the time was that any lymph node involvement was a poor prognostic factor. Subsequent surgical series suggested that the preferential pattern of lymphatic drainage in MPM is to N2 lymph nodes, including mediastinal regions such as peridiaphragmatic and internal mammary lymph nodes not usually involved in lung cancers (17,27-29). Involvement of N1 lymph nodes only was also reported to be associated with a better survival, and multiple N2 lymph node stations with a worse survival (17). By univariate analysis, our data suggest a difference in survival for N1 versus N2 disease (Figure 5), but these differences are not significant in multivariable analyses (Table 4). More information about the extent of lymph node involvement is needed to resolve this issue.
Stage groupings, especially for stages I and II disease, need to be reassessed. Univariate analyses of this database (Figures 2,3) suggest that the current stage groupings identify patient groups with distinctly different survivals. However, multivariable analyses taking into account known significant prognostic factors do not show a significant difference between stages I and II. Future analyses with more detailed information about T and N categories (as noted above) should readdress this since it is important that stage groupings be applicable across histological subtypes and patient age and sex. Although differences among stages II, III and IV remain significant in these analyses, stages III and IV define broad categories of disease, including both locally advanced tumors (T3 and T4), regionally advanced disease (N1 and N2) and metastatic disease (M1). In the future, the addition of a larger group of patients with more advanced disease, staged clinically and managed nonsurgically, may help determine whether stages III and IV should be classified into “a” or “b” subcategories.
The primary purpose of this database is to determine whether and how the current TNM staging system should be revised. Given retrospective data, heterogeneous data sources and individualized treatment selection, evaluation of the effect of clinical interventions on survival can only be considered exploratory and hypothesis-generating. Other studies, both retrospective and prospective, have suggested a beneficial effect of both “curative intent” surgery and multimodality treatment on survival (9,11,30-33). Our data, absent details of adjuvant chemotherapy and radiotherapy, are consistent with previous reports. The role of EPP versus P/D remains highly controversial with impassioned views pro and con the use of EPP (34-47). In our data set, stage I patients treated with EPP survived longer than other patients. Why they survived longer cannot be determined without some understanding of how treatment was selected for these patients. Similar to lung cancer, different surgical procedures may be appropriate for different groups of patients. It is perhaps time to study this question prospectively with more restricted stage and prognostic factor eligibility than has been done in the past.
In summary, analyses of this database suggest that the current MPM staging system does generally classify patients into groups with distinctly different outcomes but also highlights areas for potential revision. As IASLC and IMIG investigators continue to expand the database, more detailed information on T and N descriptors, as well as the addition of patients staged clinically and managed non-surgically, is required in order to propose revisions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
The following sponsor[s] provided funding to support the International Mesothelioma Staging Project: Mesothelioma Applied Research Foundation, and the International Association for the Study of Lung Cancer. Sponsor[s] had no input into the committee’s analysis of the data, nor in the committee’s suggestions for revisions to the staging system.
References
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8.
- International Union Against Cancer. eds. TNM Classification of Malignant Tumours. 7th ed. Oxford, UK: Wiley-Blackwell, 2009.
- American Joint Committee on Cancer. eds. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010.
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14.
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the International Association For the Study of Lung Cancer mesothelioma database. J Thorac Oncol 2012;7:1631-1639.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8.
- Bölükbas S, Manegold C, Eberlein M, et al. Survival after trimodality therapy for malignant pleural mesothelioma: radical pleurectomy, chemotherapy with cisplatin/ pemetrexed and radiotherapy. Lung Cancer 2011;71:75-81.
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202.
- Hasani A, Alvarez JM, Wyatt JM, et al. Outcome for patients with malignant pleural mesothelioma referred for trimodality therapy in Western Australia. J Thorac Oncol 2009;4:1010-6.
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13.
- Yamanaka T, Tanaka F, Hasegawa S, et al. A feasibility study of induction pemetrexed plus cisplatin followed by extrapleural pneumonectomy and postoperative hemithoracic radiation for malignant pleural mesothelioma. Jpn J Clin Oncol 2009;39:186-8.
- Richards WG, Godleski JJ, Yeap BY, et al. Proposed adjustments to pathologic staging of epithelial malignant pleural mesothelioma based on analysis of 354 cases. Cancer 2010;116:1510-7.
- Mineo TC, Ambrogi V, Pompeo E, et al. The value of occult disease in resection margin and lymph node after extrapleural pneumonectomy for malignant mesothelioma. Ann Thorac Surg 2008;85:1740-6.
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65.
- Yan TD, Boyer M, Tin MM, et al. Extrapleural pneumonectomy for malignant pleural mesothelioma: outcomes of treatment and prognostic factors. J Thorac Cardiovasc Surg 2009;138:619-24.
- Flores RM, Routledge T, Seshan VE, et al. The impact of lymph node station on survival in 348 patients with surgically resected malignant pleural mesothelioma: implications for revision of the American Joint Committee on Cancer staging system. J Thorac Cardiovasc Surg 2008;136:605-10.
- Cao C, Krog Andvik SK, Yan TD, et al. Staging of patients after extrapleural pneumonectomy for malignant pleural mesothelioma--institutional review and current update. Interact Cardiovasc Thorac Surg 2011;12:754-7.
- de Perrot M, Uy K, Anraku M, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2007;133:111-6.
- Liu F, Zhao B, Krug LM, et al. Assessment of therapy responses and prediction of survival in malignant pleural mesothelioma through computer-aided volumetric measurement on computed tomography scans. J Thorac Oncol 2010;5:879-84.
- Plathow C, Klopp M, Thieke C, et al. Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur Radiol 2008;18:1635-43.
- Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 2010;16:2409-17.
- Ak G, Metintas M, Metintas S, et al. Three-dimensional evaluation of chemotherapy response in malignant pleural mesothelioma. Eur J Radiol 2010;74:130-5.
- Frauenfelder T, Tutic M, Weder W, et al. Volumetry: an alternative to assess therapy response for malignant pleural mesothelioma? Eur Respir J 2011;38:162-8.
- Sensakovic WF, Armato SG 3rd, Straus C, et al. Computerized segmentation and measurement of malignant pleural mesothelioma. Med Phys 2011;38:238-44.
- Nakas A, Black E, Entwisle J, et al. Surgical assessment of malignant pleural mesothelioma: have we reached a critical stage? Eur J Cardiothorac Surg 2010;37:1457-63.
- Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg 1996;111:815- 25; discussion 825-6.
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95.
- Edwards JG, Stewart DJ, Martin-Ucar A, et al. The pattern of lymph node involvement influences outcome after extrapleural pneumonectomy for malignant mesothelioma. J Thorac Cardiovasc Surg 2006;131:981-7.
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22:3451-7.
- Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): Feasibility and results. Lung Cancer 2007;57:89-95.
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504.
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9.
- Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 2010;5:1692-703.
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.
- Martin-Ucar AE, Nakas A, Edwards JG, et al. Casecontrol study between extrapleural pneumonectomy and radical pleurectomy/decortication for pathological N2 malignant pleural mesothelioma. Eur J Cardiothorac Surg 2007;31:765-70; discussion 770-1.
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95.
- Yan TD, Cao CQ, Boyer M, et al. Improving survival results after surgical management of malignant pleural mesothelioma: an Australian institution experience. Ann Thorac Cardiovasc Surg 2011;17:243-9.
- de Perrot M, McRae K, Anraku M, et al. Risk factors for major complications after extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Thorac Surg 2008;85:1206-10.
- Aigner C, Hoda MA, Lang G, et al. Outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008;34:204-7.
- Schipper PH, Nichols FC, Thomse KM, et al. Malignant pleural mesothelioma: surgical management in 285 patients. Ann Thorac Surg 2008;85:257-64; discussion 264.
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3.
- Nakas A, Trousse DS, Martin-Ucar AE, et al. Open lungsparing surgery for malignant pleural mesothelioma: the benefits of a radical approach within multimodality therapy. Eur J Cardiothorac Surg 2008;34:886-91.
- Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6.
- Sugarbaker DJ, Wolf AS, Chirieac LR, et al. Clinical and pathological features of three-year survivors of malignant pleural mesothelioma following extrapleural pneumonectomy. Eur J Cardiothorac Surg 2011;40:298-303.
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extrapleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72.
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5.





