Systematic review of trimodality therapy for patients with malignant pleural mesothelioma
Background: Malignant pleural mesothelioma (MPM) is an aggressive form of cancer arising from the
pleural mesothelium. Trimodality therapy (TMT) involving extrapleural pneumonectomy with neoadjuvant
or adjuvant chemotherapy and adjuvant radiotherapy is a recognized treatment option with a curative intent.
Despite encouraging results from institutional studies, TMT in the treatment of MPM remains controversial.
The present systematic review aims to assess the safety and efficacy of TMT in the current literature.
Methods: A systematic review was performed using five electronic databases from 1 January 1985 to 1
October 2012. Studies were selected independently by two reviewers according to predefined selection
criteria. The primary endpoint was overall survival. Secondary endpoints included disease-free survival,
disease recurrence, perioperative morbidity and length of stay.
Results: Sixteen studies were included for quantitative assessment, including one randomized controlled
trial and five prospective series. Median overall survival ranged from 12.8-46.9 months. Disease-free survival
ranged from 10-16.3 months. Perioperative mortality ranged from 0-12.5%. Overall perioperative morbidity
ranged from 50-82.6% and the average length of stay was 9-14 days.
Conclusions: Outcomes of patients who underwent TMT in the current literature appeared to be
inconsistent. Four prospective series involving a standardised treatment regimen with neoadjuvant
chemotherapy indicated encouraging results based on intention-to-treat analysis. However, a small study
assessing the feasibility of conducting a randomized controlled trial for TMT versus conservative treatment
reported poor short- and long-term outcomes for patients who underwent pneumonectomy. Overall, results
of the present systematic review suggest TMT may offer acceptable perioperative outcomes and long-term
survival in selected patients treated in specialized centers.
Key words: Systematic review; mesothelioma; trimodality therapy; extrapleural pneumonectomy
Background
Malignant pleural mesothelioma (MPM) is an aggressive form of cancer arising from the pleural mesothelium (Figure 1). Although relatively rare, the incidence of MPM is expected to peak in many developed nations over the coming decade (1,2). Medical management of MPM has been limited by modest responses to modern chemotherapeutic agents and radiotherapy alone (3,4). Surgical options for patients with MPM can be divided into those with a palliative intent and those with a curative intent. In the latter group, patients who are deemed to have resectable disease can be offered surgical procedures such as extrapleural pneumonectomy (EPP) or extended pleurectomy/decortication (P/D) that aim to achieve macroscopic clearance and maximal cytoreduction.
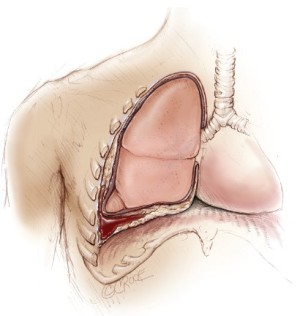
An international consensus report recently concluded that more surgeons believed EPP could provide adequate cytoreduction compared to extended P/D (90% vs. 68%) (5). In most specialised centres, EPP is now routinely performed as part of a trimodality treatment (TMT) regimen involving neoadjuvant or adjuvant chemotherapy, surgery and adjuvant radiotherapy. Despite encouraging results from institutional reports, a number of studies comparing EPP to less invasive procedures have questioned the merit of this surgical technique, and the evidence for TMT remains controversial according to current guidelines (6-8). The primary aim of the present systematic review was to assess the safety and efficacy of TMT involving EPP, and to identify its role in the surgical management of patients with MPM.
Methods
Literature search strategy
Electronic searches were performed using Ovid Medline, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club, and Database of Abstracts of Review of Effectiveness (DARE) from January 1985 to October 2012. To achieve the maximum sensitivity of the search strategy and identify all studies, we combined the terms “mesothelioma” and “pneumonectomy” as either key words or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible studies for the present systematic review included those in which patients with histologically proven MPM were treated by EPP, neoadjuvant or adjuvant chemotherapy, and adjuvant radiotherapy. All forms of systemic chemotherapy and radiotherapy were included. For studies that included patients who underwent TMT as a subset of patients who had other treatment regimens, results for patients who underwent TMT were extracted when possible. When centres have published duplicate trials with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for qualitative appraisal at each time interval. It is acknowledged that patient selection for TMT varied amongst institutions and sometimes within an institution at different time periods. All publications were limited to human subjects and in English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted due to potential publication bias and possible duplication of results. Studies that included fewer than twenty patients intended for treatment in prospective studies or who underwent EPP in retrospective studies were also excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators (D.T. and P.M.) independently reviewed each retrieved article. Discrepancies between the two reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigators (C.C. and T.D.Y.).
Results
Quantity and quality of trials
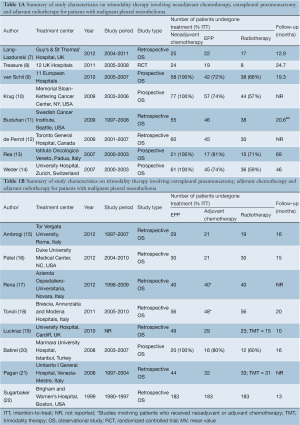
A total of 502 references were identified through the five electronic database searches. A summary of the study selection process is summarized in Figure 2. After inclusion of 12 studies from other sources and exclusion of duplicate references, 487 potentially relevant articles were retrieved for more detailed evaluation. After applying the selection criteria, 226 studies remained for assessment. Manual search of the reference lists did not identify any additional relevant studies. Full articles were obtained and further evaluated. Of the 16 studies included for final analysis in the present systematic review, one study was a feasibility-testing randomized controlled trial, 5 were prospective series, and the remainder were from retrospective observational studies, as summarized in Table 1A,1B (7-22).
Full table
In these 16 studies, 744 patients with MPM who underwent EPP were included, and 612 patients underwent TMT. Studies that only included patients who underwent neoadjuvant chemotherapy, EPP and adjuvant radiotherapy are presented separately to patients who had adjuvant chemotherapy, as summarized in Table 1A and 1B. Baseline characteristics, patient selection and follow-up periods varied between institutions.
Assessment of overall survival
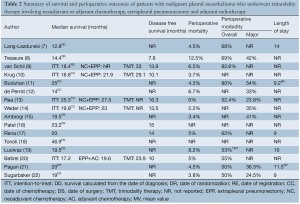
A summary of median overall survival outcomes is presented in Table 2. In studies involving neoadjuvant chemotherapy, the definition of overall survival differed between institutions, with some centres calculating survival from the date of diagnosis (7,11), randomization (8), registration (9) or commencement of chemotherapy (10,12-14). In this group, four prospective studies reported a median overall survival of 16.8-25.5 months on intention-to-treat analysis (9,10,13,14). A randomized controlled trial reported a median survival of 14.4 months from 24 patients who were randomized to undergo EPP (8). Ten patients in this trial were also included in a retrospective institutional report, which presented a median overall survival of 12.8 months from the date of diagnosis (7). In studies involving adjuvant chemotherapy, the majority of studies reported survival from the date of EPP (15,16,18,19,21,22). but not specified in others (17,20). In this group, apart from one multi-centre retrospective study reporting a median survival of 46.9 months, the remaining studies reported median survival periods of 19-24 months (15-17,19-22).
Full table
Reporting of disease-free survival (DFS) was variable between centres, and differences in the form and frequency of follow-up made interpretation difficult. In studies involving neoadjuvant chemotherapy, four prospective series reported DFS of 10.1- 16.3 months (9,10,13,14), whilst the randomized controlled trial reported 7.6 months (8). Three studies involving adjuvant chemotherapy reported DFS of 10-15 months (16,17,20).
Assessment of perioperative outcomes
A summary of perioperative outcomes, including perioperative mortality, morbidity and length of stay, is presented in Table 2. Perioperative mortality ranged from 0-12.5%. Perioperative morbidity was measured according to different grading systems in various institutions, with an overall rate of 50-83%. Major perioperative complications ranged between 24-54%. The average length of stay was reported to be 9-14 days.
Adjuvant therapy and treatment failure
In studies involving neoadjuvant chemotherapy, four prospective series had standardized regimens (9,10,13,14), whilst the remaining retrospective reports (7,11,12) and the randomized controlled trial (8) had variable treatment combinations. Studies on TMT involving adjuvant chemotherapy also reported inconsistent therapeutic regimens. The form of adjuvant radiotherapy also differed between institutions, with the introduction of intensity modulated radiotherapy (IMRT) in some centres in recent years (11,12,16,18). The reporting of local disease recurrence ranged from 4-41%, distant recurrence ranged from 5-65%, and overall disease recurrence ranged from 27-84%. However, duration and methodology of follow-up varied between institutions. A summary of chemotherapy and radiotherapy regimens and patterns of disease recurrence are presented in Table 3A,3B.
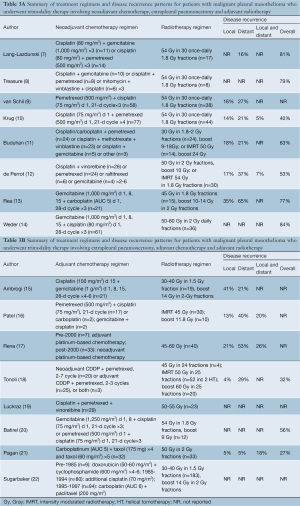
Full table
Conclusions
The necessity of adjuvant therapy with EPP was identified by Eric Butchart in the 1970s, when he stated in a personal communication that “we have recently analysed our experience with both pleuropneumonectomy and pleurectomy/decortication for mesothelioma. The very strong message from this analysis is that adjuvant therapy is essential in order to achieve any degree of long term survival with either surgical procedure” (23). In his original report of 29 patients who underwent EPP, there was a perioperative mortality rate of 31%, a perioperative morbidity rate of 45%, and a median survival of merely 10 months (24). Since then, improvements in surgical techniques and perioperative care, as well as advances in medical and radiation oncology, have resulted in significantly superior outcomes in patients treated by TMT (10,12). In addition, an improved understanding of the important prognostic factors and a refinement of the patient selection process have helped to identify patients who may benefit most from aggressive therapy and avoid futile treatment in patients who are unsuitable surgical candidates (25).
Although TMT involving EPP, adjuvant chemotherapy and radiotherapy has been utilized since the introduction of EPP for selected patients with MPM, the first prospective study assessing the safety and efficacy of a standardized TMT program involving neoadjuvant chemotherapy, EPP and adjuvant radiotherapy was not published until 2004 (26). In their pilot study, Weder and colleagues treated 19 patients with neoadjuvant gemcitabine and cisplatin, followed by EPP in 16 patients and adjuvant radiotherapy in 13 patients. The authors stated that the neoadjuvant chemotherapeutic approach offered both logistical and biological advantages compared to the adjuvant chemotherapy regimen. This was reflected by a reported median survival of 23 months by an intention-to-treat analysis, which was far superior to a median survival of 10 months during their previous treatment protocol involving EPP followed by adjuvant chemotherapy and radiotherapy. Subsequently, a larger cohort of patients from the same investigators replicated these early encouraging outcomes (14).
Over the last decade, a number of improvements have been developed in adjuvant therapy for MPM in combination with EPP. A randomized controlled trial by Vogelzang et al. has established pemetrexed and cisplatin as the accepted first-line chemotherapy regimen for MPM in many centres (3,27). More recently, the emergence of biological and immunotherapy agents have gained intense interest, with a number of Phase I trials currently under investigation (28,29). Advances in radiation oncology in the form of IMRT have resulted in further improvements in overall survival (11,16,27,30). Compared to other surgical options, EPP allows higher doses of radiotherapy to the hemithorax by avoiding pulmonary toxicity, and this approach has demonstrated a significant reduction in loco-regional relapses (30). Modern techniques such as helical tomography allow the delivery of superior dose homogeneity in the target volume whilst minimizing radiation to the normal critical structures such as the spinal cord, heart, oesophagus and liver. The improved outcomes of patients who underwent TMT involving modern radiation oncology treatment was presented by Tonoli and colleagues, who reported a median survival of 46.9 months for patients who underwent IMRT with a median dose of 52 Gy (18).
Overall, the present systematic review identified 16 studies on TMT involving neoadjuvant or adjuvant chemotherapy, EPP and adjuvant radiotherapy according to predefined criteria, including one randomized controlled trial and five prospective series. Studies on TMT involving EPP, adjuvant chemotherapy and radiotherapy were mostly retrospective reports with non-standardized chemotherapy regimens. In comparison, studies involving neoadjuvant chemotherapy, EPP and adjuvant radiotherapy were relatively more recent, mostly including patients treated after year 2000. Four prospective studies involving patients treated by a standardized neoadjuvant chemotherapy regimen, EPP and adjuvant radiotherapy reported a median survival of 16.8-25.5 months, with a perioperative mortality of 0-5% (9,10,13,14). In these four studies, the majority of patients (57-71%) were able to complete the trimodality therapy on an intention-to-treat analysis. In contradistinction, the Mesothelioma and Radical Surgery (MARS) trial reported a median survival of 14.4 months for the 24 patients randomized to undergo EPP, of whom 10 were reported in another study by Lang-Lazdunski et al. (7,8) Investigators of the MARS trial pointed out that survival outcomes were calculated from the later timepoint of randomization, with a median period of 3.6 months after the registration process. The primary intent of this trial was to assess the feasibility of conducting a randomized controlled trial for EPP in the management of MPM. However, the study design and subsequent data analysis have met significant criticism (31,32). Major points of contention include the speculative conclusions drawn from a feasibility-testing study, the non-standardized chemotherapy agents and timing of chemoradiation, limited numbers of recruited patients and significant protocol violations between the two treatment arms. Indeed, with a mortality rate of 18% for the 17 patients who underwent EPP per protocol, it represents one of the highest mortality rates for EPP in the current literature (27,31). Despite this, investigators from the MARS trial are considered to have the unique opportunity to perform a definitive therapeutic trial that could assess the efficacy of EPP compared to medical management (32). Of the remaining studies within the present systematic review, the perioperative mortality ranged from 0-8.2%, with a wide range of perioperative morbidity and disease recurrence outcomes, partly due to non-standardized reporting.
In conclusion, the present systematic review has shown that EPP for patients with MPM can be performed with an acceptable perioperative mortality rate in specialized centres. However, the evidence for long-term survival in patients treated by TMT in the current literature is inconsistent. A number of prospective studies with standardized therapeutic regimens have reported relatively favourable outcomes on intention-to-treat analysis. These encouraging results demonstrate the potential benefit TMT can offer for patients treated by a multi-disciplinary approach in well-integrated programs. Conversely, one randomized controlled trial reported relatively poor perioperative and long-term outcomes for patients randomized to EPP (8). Investigators of the MARS trial should be commended for their efforts in demonstrating the feasibility of conducting a randomized study on EPP versus conservative treatment. However, further evidence is required before definitive conclusions can be drawn about the efficacy of this surgical procedure. the present study is limited by potential publication bias, and it should be acknowledged that the majority of data presented have been obtained from tertiary centres with a special interest in the surgical management of MPM. Hence, these results should be interpreted with caution and may not be applicable to non-specialized institutions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603.
- Clements M, Berry G, Shi J, et al. Projected mesothelioma incidence in men in New South Wales. Occup Environ Med 2007;64:747-52.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
- Waite K, Gilligan D. The role of radiotherapy in the treatment of malignant pleural mesothelioma. Clin Oncol (R Coll Radiol) 2007;19:182-7.
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma: a consensus report of the International Association for the Study of Lung Cancer International Staging Committee and the International Mesothelioma Interest Group. J Thorac Oncol 2011;6:1304-12.
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95.
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/ decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43.
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extrapleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72.
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9.
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13.
- Buduhan G, Menon S, Aye R, et al. Trimodality therapy for malignant pleural mesothelioma. Ann Thorac Surg 2009;88:870-5; discussion 876.
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8.
- Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): Feasibility and results. Lung Cancer 2007;57:89-95.
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202.
- Ambrogi V, Baldi A, Schillaci O, et al. Clinical impact of extrapleural pneumonectomy for malignant pleural mesothelioma. Ann Surg Oncol 2012;19:1692-9.
- Patel PR, Yoo S, Broadwater G, et al. Effect of increasing experience on dosimetric and clinical outcomes in the management of malignant pleural mesothelioma with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:362-8.
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5.
- Tonoli S, Vitali P, Scotti V, et al. Adjuvant radiotherapy after extrapleural pneumonectomy for mesothelioma. Prospective analysis of a multi-institutional series. Radiother Oncol 2011;101:311-5.
- Luckraz H, Rahman M, Patel N, et al. Three decades of experience in the surgical multi-modality management of pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:552-6.
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504.
- Pagan V, Ceron L, Paccagnella A, et al. 5-year prospective results of trimodality treatment for malignant pleural mesothelioma. J Cardiovasc Surg (Torino) 2006;47:595-601.
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5.
- Treasure T, Utley M. Ten traps for the unwary in surgical series: a case study in mesothelioma reports. J Thorac Cardiovasc Surg 2007;133:1414-8.
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24.
- Cao C, Yan TD, Bannon PG, et al. Summary of prognostic factors and patient selection for extrapleural pneumonectomy in the treatment of malignant pleural mesothelioma. Ann Surg Oncol 2011;18:2973-9.
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22:3451-7.
- Cao CQ, Yan TD, Bannon PG, et al. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol 2010;5:1692-703.
- Bograd AJ, Suzuki K, Vertes E, et al. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol Immunother 2011;60:1509-27.
- Hassan R, Cohen SJ, Phillips M, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res 2010;16:6132-8.
- Chi A, Liao Z, Nguyen NP, et al. Intensity-modulated radiotherapy after extrapleural pneumonectomy in the combined-modality treatment of malignant pleural mesothelioma. J Thorac Oncol 2011;6:1132-41.
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4; author reply 1094-5.
- Rusch VW. The Mars trial: resolution of the surgical controversies in mesothelioma? J Thorac Oncol 2009;4:1189-91.





