Chemotherapy for malignant pleural mesothelioma: a review of current management and a look to the future
The systemic treatment of malignant pleural mesothelioma (MPM) in routine practice has remained unchanged since 2003, with almost a decade passing since any new treatment being approved for this disease. However, there are currently more novel agents in clinical trials than ever before, bringing hope that the next decade will see improvements in survival and other patient outcomes. This review will focus on systemic treatments for advanced disease, with an overview of current practice and a view to the future of chemotherapy and targeted therapies in mesothelioma. The use of chemotherapy as part of multimodality treatment for mesothelioma is covered in a companion paper in this issue and will not be addressed here.
First-line chemotherapy
Despite decades of clinical research, cytotoxic chemotherapy remains one of the few therapeutic options that has been proven to improve survival in patients with MPM in a randomised controlled trial (Figure 1). Readers will be familiar with the pivotal ‘Emphacis’ trial which demonstrated that the combination of cisplatin and pemetrexed gave a three month survival benefit over cisplatin alone, improving median survival from 9.3 to 12.1 months (P=0.02) in patients with advanced disease (1). This modest survival increase was also associated with improvements in quality of life (2). A similar survival benefit was seen with the addition of raltitrexed to cisplatin, with survival increasing from 8.8 to 11.4 months, although objective radiological response rates were lower to this combination than to cisplatin and pemetrexed (3). Whilst neither of these trials included a comparator arm of Best Supportive Care, the British MS01 study did randomise patients to active symptom control (ASC) with or without either vinorelbine or MVP (mitomycin C, vinblastine, and cisplatin) (4). This trial accrued poorly, and after closing early, both chemotherapy arms were combined for analysis of the primary endpoint; no survival benefit was seen overall for the combined chemotherapy arms as compared with ASC (HR 0.89; P=0.29). Nevertheless, when the two arms were analysed independently, there was a substantial difference between them with a two month survival benefit for vinorelbine over ASC nearing statistical significance (HR 0.80, P=0.08), whilst MVP did not give a signal for benefit (HR 0.99, P=0.95). Although no trial has demonstrated a benefit of platinum and an antifolate over supportive care, the weight of the evidence suggests that an active platinum-based combination is likely to give a benefit of at least three months over best supportive care.
On the basis of these data, the combination of cisplatin and pemetrexed has become standard first-line therapy worldwide for patients who are not suitable for aggressive surgery, or in whom chemotherapy is recommended as part of a multimodality regimen. Carboplatin is often substituted for cisplatin, due to simpler and shorter administration and a perception of lesser toxicity. Although carboplatin use is not supported by randomised evidence, and there has been no direct comparison between the two platinum agents, phase I and II studies have demonstrated similar activity of either carboplatin or cisplatin with pemetrexed, with objective radiological response rates between 20% and 30% (5,6). Most importantly, although an expanded access program showed a slightly lower response rate for carboplatin based therapy, one year survival and time to progression were very similar (7). Most oncologists will substitute carboplatin for cisplatin on the basis of clinical judgement, for example where patients have medical contraindications to cisplatin.
Despite the use of cisplatin or carboplatin with pemetrexed as first-line therapy for advanced mesothelioma for nearly 10 years, many practical questions remain in our use of chemotherapy in mesothelioma. Some of these uncertainties have been answered in non-small cell lung cancer (NSCLC), and whilst the numbers of patients with mesothelioma would be sufficient for international trial efforts to answer these questions, they have not been (and may never be) a high priority over novel treatment trials. In one example, treatment of non-small cell lung cancer has recently moved to incorporate a maintenance phase following primary chemotherapy, a strategy which has been supported by a number of randomised clinical trials and meta-analyses (8-11). In mesothelioma, appropriate randomised studies have not yet been done, although a small study has demonstrated the safety and feasibility of continuing single agent pemetrexed (12). A phase II trial randomising patients to continue single agent pemetrexed or observation only is underway (NCT01085630) but is not likely to report for another two years. Similarly, it will probably remain unclear whether 4 cycles of chemotherapy provides similar survival benefits with lesser toxicity than 6 cycles or ongoing chemotherapy, as shown in NSCLC (13). Finally, in asymptomatic patients who would be suitable for cytotoxic chemotherapy and will not be having surgical management, should we start chemotherapy immediately on diagnosis, or delay treatment until the development of symptoms and measurable disease? One small randomised trial of 43 patients using the inactive MVP regimen suggested a survival benefit for immediate treatment, however the lack of activity of MVP when compared with Active Symptom Control makes this result unconvincing (14), and the question remains unanswered.
If we recommend platinum and pemetrexed chemotherapy, how can we best select our patients and predict for response to therapy? In non-small cell lung cancer, tumor histology is an important determinant of response, with squamous cell carcinoma being highly resistant to pemetrexed (15). Pemetrexed is a multitargeted antifolate agent, inhibiting thymidylate synthetase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). Resistance to pemetrexed in squamous cell carcinoma appears to be mediated by high levels of TS present in this histological subtype. There is some indication that this may be relevant for mesothelioma, with a small study finding a significant correlation between low TS protein expression on immunohistochemistry and time to progression (TTP) and overall survival (OS) in patients treated with pemetrexed, which was not seen in untreated patients (16). However, this finding is unconfirmed and should not be used to guide management in individual patients. Although sarcomatoid histology, found in approximately 10% of patients, has invariably been found to predict poor prognosis, neither randomised trials showing survival benefits for combination chemotherapy reported on an interaction between efficacy and tumor subtype (1,3). The cytotoxicity of cisplatin and carboplatin is mediated through platinum-DNA adult formation, which can be repaired by excision repair cross-complementing 1 (ERCC1) and other enzymes in the nucleotide excision repair pathway. Although low levels of ERCC1 predict for potential benefit from platinum-based therapy in NSCLC, this relationship has not been borne out in mesothelioma, with high ERCC1 protein expression appearing to predict for survival benefit, irrespective of treatment (16). Finally, the alpha folate receptor is over-expressed by most mesotheliomas, but did not appear to correlate with response to pemetrexed in vitro and is not known to be useful in patient selection (17,18).
Other prognostic indicators which do not yet appear to have a role in predicting response to systemic therapy include serum mesothelin levels, 18F-fluorodeoxyglucose Positron Emission Tomography (FDG-PET) at baseline, and molecular tests. Serum mesothelin levels reflect disease burden and predict survival, also decreasing with response to treatment and increasing with disease progression. However, there is no indication that elevated mesothelin levels are predictive of treatment response (19). Quantitative baseline FDG-PET parameters, notably total glycolytic volume (TGV) and to a lesser extent maximum standardised uptake value (SUVmax), are also prognostic indicators, at least in those with non-sarcomatoid disease (20). However, again, baseline FDG-PET parameters do not appear predictive of treatment response. A four-gene expression ratio test performed better than histological subtype, tumor stage, and lymph node status as a predictor of surgical outcomes, but has not been reported as a predictor of outcomes on systemic therapy (21).
Second-line chemotherapy
After initial chemotherapy, or aggressive multimodality therapy, patients almost invariably experience disease recurrence or progression. Many patients will be fit for, and may want, second-line chemotherapy at this point. The only randomised clinical trial in this setting showing an improvement in progression free survival (PFS) was undertaken before the widespread use of pemetrexed as first-line treatment. This study compared second-line pemetrexed versus best supportive care, with a significantly higher rate of partial response, disease control, and longer PFS in the group receiving pemetrexed (22). However, there is no randomised trial testing the use of pemetrexed as re-treatment following previous first-line use. Nevertheless, a recent retrospective review of second-line chemotherapy found that disease control with second-line therapy was better in those patients who received pemetrexed, and those with a prolonged time to progression (≥12 months) after first-line therapy (23). Furthermore, patients re-treated with a platinum-pemetrexed combination had a lower risk of death than those treated with pemetrexed alone (HR =0.11, P<0.001), although this observation may be confounded by selection bias in this non-randomised comparison, with fitter patients potentially more likely to receive combination therapy. Together, this suggests that re-treatment with pemetrexed and a platinum is a reasonable option for second-line therapy in fit patients with previous disease control after pemetrexed-based treatment.
A variety of cytotoxic agents have some activity in second-line treatment, but none have had the appropriate randomised controlled design to satisfy regulators or clinicians that any particular agent is the best choice. The lack of randomised designs also means that we do not know if survival benefits accrue from agents with modest objective radiological responses in the second-line setting. This also leads to a conundrum in the design of randomised second-line trials of novel agents: whilst patients commonly receive off-label second-line therapy, this is neither approved by regulatory authorities, nor standardised, making the choice of chemotherapy drug or placebo as a comparator arm open to debate. Single agent vinorelbine has a response rate (RR) of 16% and overall survival of 9.6 months at second-line (24). Combinations of gemcitabine and vinorelbine (RR 10%, OS 10.9 months, PFS 2.8 months) (25), gemcitabine and epirubicin (RR 13%, OS 9.3 months, PFS 6.3 months in a high dose group) (26), irinotecan, cisplatin, and mitomycin (RR 20%, OS 7.3 months, PFS 7.3 months) (27) and others have been reported. On balance, the tolerability and response rate of single agent vinorelbine has been favoured in practice and as a proposed control arm for a new generation of second-line trials. However retrospective data presented at the recent International Mesothelioma Interest Group meeting failed to confirm a meaningful rate of objective responses in routine clinical practice (Zauderer M, personal communication, September 2012). The second-line setting is still waiting for a new agent, which can demonstrate both responses and survival benefits in a randomised trial.
Systemic therapy in the era of precision medicine
The paradigm for drug development in the last century was empirical testing of new drugs in clinical trials, usually with in vitro or in vivo activity rather than specific molecular targets as the rationale for testing. In the current century, a very different paradigm of therapeutic discovery has evolved, and is relying on genomic medicine to identify targets which can then be translated to the clinic. Mesothelioma, like other complex adult cancers, evolves through a multistep process of carcinogenesis involving genetic and epigenetic changes. Some of these genetic changes will be passenger mutations, which are present but do not confer a growth advantage. Others, however, are likely to be the important driver mutations which have been positively selected and are key to the pathogenesis of the tumor. Such mutations may be either ‘actionable’ or ‘druggable’. Actionable mutations are those which contribute to our understanding of an individual’s cancer in terms of prognosis or treatment selection. Druggable mutations are the holy grail of cancer therapeutic discovery, and are those for which drugs are available to specifically target the mutation. Unfortunately, no clearly actionable or druggable activating mutations or translocations have yet been identified in mesothelioma, and certainly none have been translated through to the clinic. However as we have seen from other cancers - for example, the recent discovery of the EML4-ALK fusion protein and the use of the ALK inhibitor crizotinib in non-small cell lung cancer - therapeutic strategies in clearly druggable targets can be fast tracked to the clinic (28).
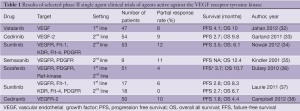
Nevertheless, over the past 10 years, a number of novel targeted agents which have shown activity in other diseases have been trialled empirically in mesothelioma, including epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) and multi-targeted TKIs with effects on the vascular endothelial growth factor (VEGF) pathway amongst others. Single agent treatment with the EGFR TKIs gefitinib, and erlotinib, as well as the PDGFR β/C-Kit inhibitor imatinib showed no evidence of activity even as first-line treatment (29-31). Agents inhibiting the VEGF receptor, often in combination with other targets, have shown some evidence of activity in an unselected population, with response rates around 10% and PFS between 3 and 4 months (32-38) (Table 1). However, identifying predictors of benefit from these agents has proven difficult, with no predictive factors identified in extensive serum testing of the VEGF pathway in one recent study of sunitinib (33). The anti-VEGF antibody bevacizumab has been tested in a number of completed and ongoing trials, with the best evidence coming from a randomised phase II trial of bevacizumab in combination with cisplatin and pemetrexed chemotherapy which did not show improved PFS or OS, although subset analyses suggested that a low serum VEGF level may predict for improved outcomes on the combination (39). The combination of cisplatin and gemcitabine is no longer a standard first-line treatment in MPM, so the ongoing French MAPS study, which has to date randomised more than 300 of a planned 445 patients to either cisplatin and pemetrexed with placebo or bevacizumab, should clarify the use of this drug in combination with chemotherapy in around two years (Scherpereel, A. Personal communication, September 2012).
Full table
Important new targets in mesothelioma have been discussed in companion papers in this journal, with mesothelioma being molecularly characterised particularly by the loss of tumor suppressor genes, rather than gain of function mutations. The recent observation that BRCA associated protein 1 (BAP1) is inactivated in around a quarter of mesothelioma tumors has raised the possibility that this subset may harbour a therapeutic target, although a number of different mutations were identified (40). BAP1 has a role in DNA repair, control of gene expression through histone modification, and enhancing progression through the G1-S checkpoint (41). The role of BAP1 in histone modification is of interest as it raises the theoretical possibility that histone deacetylase inhibitors (HDACi) may have activity in this disease, however the lack of clinical response in a recent large (n=660) randomised phase III trial of the HDACi vorinostat argues against this as an important therapeutic strategy in tumors with BAP1 loss, even in a subset of patients (42).
Another tumor suppressor gene which is frequently inactivated in this disease is neurofibromatosis type 2 (NF2), with NF2 loss occurring in around 40% of patients, mostly a different subset to those with BAP1 loss (40,43,44). NF2 encodes for the protein Merlin, which in turn interacts with more than 30 other intracellular proteins. Key pathways which may be open to manipulation are the Hpo (Hippo) pathway which is important in cell proliferation, the activation of mTORC1 by Merlin loss (45), the activation of the focal adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK) pathways, and the role of Merlin loss in removing inhibition of CRL4, a ubiquitin ligase, thus allowing broad dysregulation of transcription (46). Drug classes which have the potential for activity on the basis of these alterations include Mammalian Target of Rapamycin (mTOR) inhibitors, and the use of dual PI3K/mTOR inhibitors in view of the compensatory upregulation of PI3K seen with mTOR inhibition alone. A phase I trial of the PI3K/mTOR inhibitor GDC0980 demonstrated activity in a subset of patients with mesothelioma (47), with an expansion cohort with this disease now fully accrued and encouraging preliminary results reported at the recent International Mesothelioma Interest Group meeting (Kindler HL, personal communication, September 2012). The role of FAK inhibitors is similarly under study, due to the negative regulation of FAK by an intact Merlin protein via FAK phosphorylation.
The final pathway I will discuss in this incomplete overview is the hepatocyte growth factor (HGF)/c-Met pathway. C-Met receptor tyrosine kinase is overexpressed in the majority of mesothelioma and has been implicated in mesothelioma cell line growth, as well as successfully inhibited with both small molecular tyrosine kinase inhibitors, and siRNA, in in vitro experiments (48). Furthermore, combined inhibition of c-Met with EGFR may be better than either strategy alone in suppressing mesothelioma cell line growth (49). MET inhibitors are under development in a number of tumor types, and this pathway is relevant to test in the clinic in this disease.
Conclusions
These examples are not a comprehensive review of the numerous potential pathways which could be targeted in mesothelioma. However, equally important is the question of how best to bring agents to the clinic, and how to design clinical trials with the potential to show a signal of activity. In the maintenance and second-line setting, there is still a role for single agent testing. In the absence of routine maintenance treatment in mesothelioma, randomisation to a study drug versus placebo is appropriate, whilst a single-arm study would be difficult if not impossible to interpret. In the second-line or subsequent setting, either a single arm study with response as an endpoint, or preferably as a randomised phase II trial, will allow for evaluation of a signal for activity. There is no doubt that combination trials using cisplatin and pemetrexed with a novel agent need to include a randomisation arm to chemotherapy alone, or risk being uninterpretable; a single arm combination study is only appropriate if designed as a phase I trial. Key to the development of the next generation of agents in mesothelioma is the collection of robust correlative biospecimens, preferably as both tissue and serum or plasma. How else can we move the science of mesothelioma treatment forward rapidly? There is little doubt that advancing mesothelioma to the next stage of precision medicine will require an international co-operative effort and extensive high-quality well annotated tissue collections. The surgical fraternity can play a key role in development of mesothelioma drug therapeutics by collecting and clinically annotating appropriate tissue specimens from patients undergoing radical surgery, diagnostic procedures, or taking part in clinical trials, and collaborating with basic scientists and oncologists in this work.
Acknowledgements
The author thanks Tracy Hayward for assistance with submission.
Disclosure: Eli Lilly Australia: travel funding, advisory board; Roche Australia: research funding, advisory board; Boehringer Ingelheim Australia: travel funding, advisory board; Verastem: advisory board; Roche International: advisory board.
References
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
- Gralla RJ, Hollen PJ, Liepa AM, et al. Improving quality of life in patients with malignant pleural mesothelioma: Results of the randomized pemetrexed + cisplatin vs. cisplatin trial using the LCSS-meso instrument. Proc Am Soc Clin Oncol 2003;22:abstr 2496.
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9.
- Muers MF, Fisher P, Snee M, et al. A randomized phase III trial of active symptom control (ASC) with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma: First results of the Medical Research Council (MRC)/British Thoracic Society (BTS) MS01 trial. J Clin Oncol 2007;25:LBA7525.
- Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 2006;24:1443-8.
- Ceresoli GL, Castagneto B, Zucali PA, et al. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer 2008;99:51-6.
- Santoro A, O’Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol 2008;3:756-63.
- Zhang X, Zang J, Xu J, et al. Maintenance therapy with continuous or switch strategy in advanced non-small cell lung cancer: a systematic review and meta-analysis. Chest 2011;140:117-26.
- Petrelli F, Borgonovo K, Cabiddu M, et al. Erlotinib as maintenance therapy in patients with advanced non-small cell lung cancer: a pooled analysis of three randomized trials. Anticancer Drugs 2011;22:1010-9.
- Behera M, Owonikoko TK, Chen Z, et al. Single agent maintenance therapy for advanced stage non-small cell lung cancer: a meta-analysis. Lung Cancer 2012;77:331-8.
- Qi WX, Tang LN, He AN, et al. Erlotinib and pemetrexed as maintenance therapy for advanced non-small-cell lung cancer: a systematic review and indirect comparison. Curr Med Res Opin 2012;28:643-50.
- van den Bogaert DP, Pouw EM, van Wijhe G, et al. Pemetrexed maintenance therapy in patients with malignant pleural mesothelioma. J Thorac Oncol 2006;1:25-30.
- Socinski MA, Schell MJ, Peterman A, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol 2002;20:1335-43.
- O’Brien ME, Watkins D, Ryan C, et al. A randomised trial in malignant mesothelioma (M) of early (E) versus delayed (D) chemotherapy in symptomatically stable patients: the MED trial. Ann Oncol 2006;17:270-5.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51.
- Righi L, Papotti MG, Ceppi P, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol 2010;28:1534-9.
- Bueno R, Appasani K, Mercer H, et al. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;121:225-33.
- Nutt JE, Razak AR, O’Toole K, et al. The role of folate receptor alpha (FRalpha) in the response of malignant pleural mesothelioma to pemetrexed-containing chemotherapy. Br J Cancer 2010;102:553-60.
- Creaney J, Francis RJ, Dick IM, et al. Serum soluble mesothelin concentrations in malignant pleural mesothelioma: relationship to tumor volume, clinical stage and changes in tumor burden. Clin Cancer Res 2011;17:1181-9.
- Nowak AK, Francis RJ, Phillips MJ, et al. A novel prognostic model for malignant mesothelioma incorporating quantitative FDG-PET imaging with clinical parameters. Clin Cancer Res 2010;16:2409-17.
- Gordon GJ, Dong L, Yeap BY, et al. Four-gene expression ratio test for survival in patients undergoing surgery for mesothelioma. J Natl Cancer Inst 2009;101:678-86.
- Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 2008;26:1698-704.
- Zucali PA, Simonelli M, Michetti G, et al. Second-line chemotherapy in malignant pleural mesothelioma: results of a retrospective multicenter survey. Lung Cancer 2012;75:360-7.
- Stebbing J, Powles T, McPherson K, et al. The efficacy and safety of weekly vinorelbine in relapsed malignant pleural mesothelioma. Lung Cancer 2009;63:94-7.
- Zucali PA, Ceresoli GL, Garassino I, et al. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer 2008;112:1555-61.
- Okuno SH, Delaune R, Sloan JA, et al. A phase 2 study of gemcitabine and epirubicin for the treatment of pleural mesothelioma: a North Central Cancer Treatment Study, N0021. Cancer 2008;112:1772-9.
- Fennell DA, Steele JP, Shamash J, et al. Efficacy and safety of first- or second-line irinotecan, cisplatin, and mitomycin in mesothelioma. Cancer 2007;109:93-9.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703.
- Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol 2007;25:2406-13.
- Govindan R, Kratzke RA, Herndon JE 2nd, et al. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res 2005;11:2300-4.
- Mathy A, Baas P, Dalesio O, et al. Limited efficacy of imatinib mesylate in malignant mesothelioma: a phase II trial. Lung Cancer 2005;50:83-6.
- Jahan T, Gu L, Kratzke R, et al. Vatalanib in malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B (CALGB 30107). Lung Cancer 2012;76:393-6.
- Garland LL, Chansky K, Wozniak AJ, et al. Phase II study of cediranib in patients with malignant pleural mesothelioma: SWOG S0509. J Thorac Oncol 2011;6:1938-45.
- Nowak AK, Millward MJ, Creaney J, et al. A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol 2012;7:1449-56.
- Kindler HL, Vogelzang NJ, Chien K, et al. SU5416 in Malignant Mesothelioma: a University of Chicago Phase II Consortium Study. Proc Am Soc Clin Oncol 2001;20:abstr 1359.
- Dubey S, Jänne PA, Krug L, et al. A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukemia Group B 30307. J Thorac Oncol 2010;5:1655-61.
- Laurie SA, Gupta A, Chu Q, et al. Brief report: a phase II study of sunitinib in malignant pleural mesothelioma. the NCIC Clinical Trials Group. J Thorac Oncol 2011;6:1950-4.
- Campbell NP, Kunnavakkam R, Leighl N, et al. Cediranib in patients with malignant mesothelioma: A phase II trial of the University of Chicago Phase II Consortium. Lung Cancer 2012;78:76-80.
- Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012;30:2509-15.
- Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011;43:668-72.
- Ventii KH, Devi NS, Friedrich KL, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008;68:6953-62.
- Krug LM, Kindler HL, Calvert H, et al. VANTAGE 014: Vorinostat in patients with advanced malignant pleural mesothelioma (MPM) previously treated with pemetrexed and either cisplatin or carboplatin therapy: a phase III, rando-mized, double-blind, placebo-controlled trial. ESMO 2011, abstract 3BA.
- Sekido Y, Pass HI, Bader S, et al. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res 1995;55:1227-31.
- Bianchi AB, Mitsunaga SI, Cheng JQ, et al. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci U S A 1995;92:10854-8.
- Sekido Y. Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci 2010;101:1-6.
- Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell 2010;140:477-90.
- Wagner AJ, Bendell JC, Dolly S, et al. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. J Clin Oncol 2011;29:abstr 3020.
- Jagadeeswaran R, Ma PC, Seiwert TY, et al. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res 2006;66:352-61.
- Kawaguchi K, Murakami H, Taniguchi T, et al. Combined inhibition of MET and EGFR suppresses proliferation of malignant mesothelioma cells. Carcinogenesis 2009;30:1097-105.





