Published online Feb 7, 2017. doi: 10.3748/wjg.v23.i5.919
Peer-review started: September 26, 2016
First decision: October 28, 2016
Revised: October 29, 2016
Accepted: November 16, 2016
Article in press: November 16, 2016
Published online: February 7, 2017
In patients undergoing pancreaticoduodenectomy (PD), unrecognized hemodynamically significant celiac axis (CA) stenosis impairs hepatic arterial flow by suppressing the collateral pathways supplying arterial flow from the superior mesenteric artery and leads to serious hepatobiliary complications due to liver and biliary ischemia, with a high rate of mortality. CA stenosis is usually due to an extrinsic compression by a previously asymptomatic median arcuate ligament (MAL). MAL is diagnosed by computerized tomography in about 10% of the candidates for PD, but only half are found to be hemodynamically significant during the gastroduodenal artery clamping test with Doppler assessment, which is mandatory before any resection. MAL release is usually efficient to restore an adequate liver blood inflow and prevent ischemic complications. In cases of failure in MAL release, postponed PD with secondary stenting of the CA and reoperation for PD should be considered as an alternative to immediate hepatic artery reconstruction, which involves the risk of postoperative thrombosis of the arterial reconstruction. We recently used this two-stage strategy in a patient undergoing surgery for pancreatic adenocarcinoma.
Core tip: In patients undergoing pancreaticoduodenectomy (PD), hemodynamically significant celiac axis (CA) stenosis has the potential to cause vascular insufficiency leading to serious hepatobiliary complications with a high rate of mortality. CA stenosis is usually due to an extrinsic compression by a previously asymptomatic median arcuate ligament (MAL). MAL release is usually efficient to restore an adequate liver blood inflow and prevent ischemic complications. In cases of failure in MAL release, postponed PD with secondary stenting of the CA and reoperation for PD should be considered as an alternative to immediate hepatic artery reconstruction, which involves the risk of postoperative thrombosis.
- Citation: Guilbaud T, Ewald J, Turrini O, Delpero JR. Pancreaticoduodenectomy: Secondary stenting of the celiac trunk after inefficient median arcuate ligament release and reoperation as an alternative to simultaneous hepatic artery reconstruction. World J Gastroenterol 2017; 23(5): 919-925
- URL: https://www.wjgnet.com/1007-9327/full/v23/i5/919.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i5.919
Pancreaticoduodenectomy (PD) involves a division of the gastroduodenal artery (GDA) and resection of the pancreaticoduodenal arcades, which depend on both the GDA and the superior mesenteric artery (SMA). In the case of hemodynamically significant celiac axis (CA) stenosis, PD suppresses the collateral pathways supplying arterial flow from the SMA into the branches of the CA and impairs hepatic arterial flow. Thus, in patients undergoing PD, CA stenosis has the potential to cause vascular insufficiency leading to serious hepatobiliary complications with a high rate of mortality[1-9]. With more than 95% accuracy, multidetector computed tomography (CT) with routine arterial reconstruction and sagittal views is currently the standard examination to allow for preoperative detection of CA stenosis[1,9-12]. Most cases of CA stenosis are related to an extrinsic compression by the median arcuate ligament (MAL). MAL is diagnosed by CT in about 10% of the candidates for PD, but only half are found to be hemodynamically significant during the GDA clamping test with Doppler assessment, which is mandatory before any resection[1,12-15]. MAL release is usually successful in restoring liver blood flow and preventing ischemic complications[1,2,9]. In cases of failure in MAL release, postponed PD with secondary stenting of the CA and reoperation for PD should be considered as an alternative to immediate hepatic artery reconstruction, which involves the risk of postoperative thrombosis[1,16-21]. We recently used this strategy in a patient undergoing surgery for pancreatic adenocarcinoma.
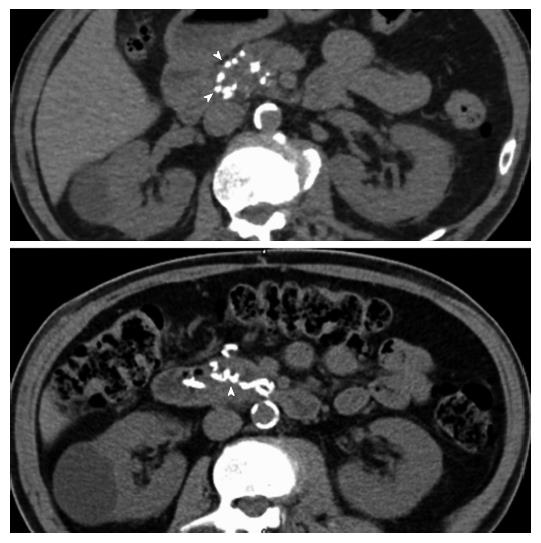
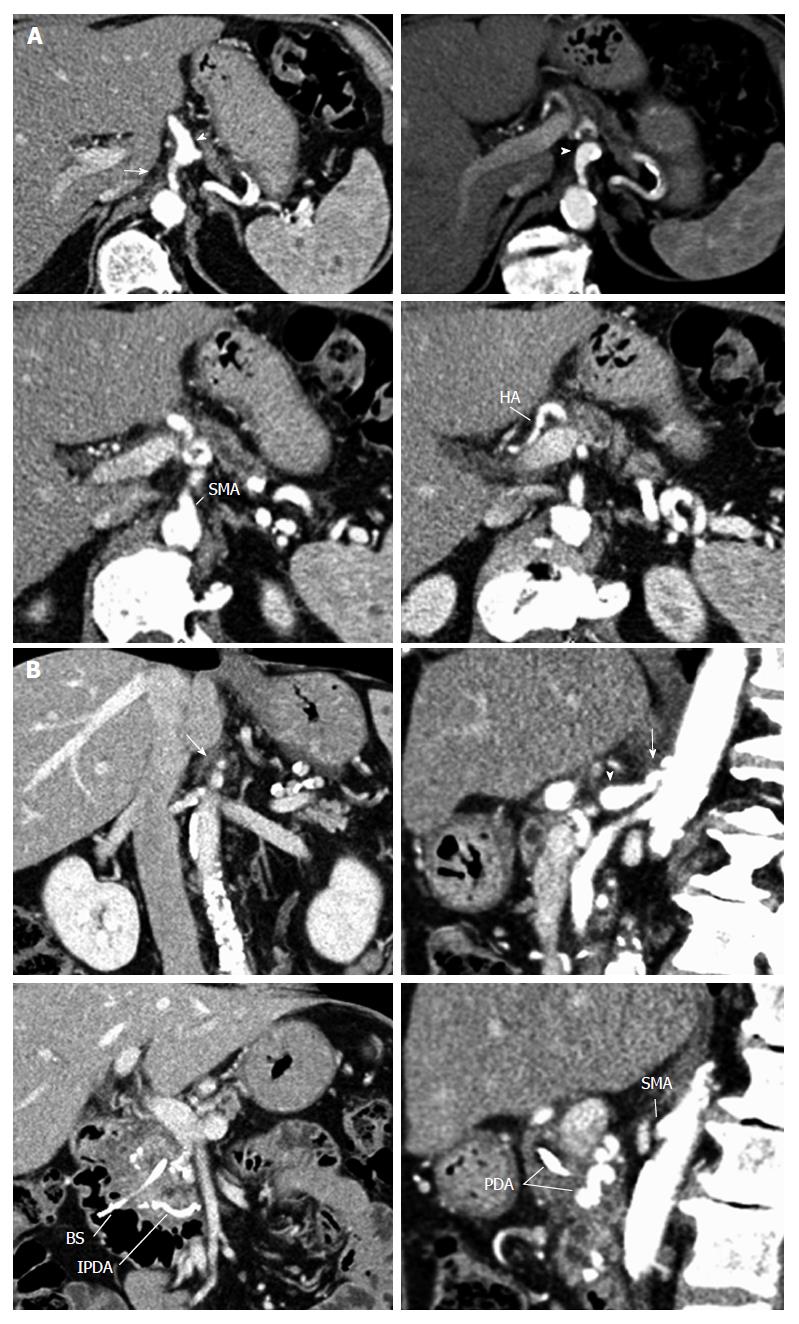
A 68-year-old male patient, presenting with insulin-requiring diabetes which evolved over 30 years, was referred with a pancreatic cephalic ductal adenocarcinoma after prosthetic drainage for jaundice and biopsy by echoendoscopy. Thoracic and abdominal CT showed no metastases. Liver magnetic resonance imaging was normal. According to the guidelines of the National Comprehensive Cancer Network, the tumor was considered resectable[22]. Abdominal CT without contrast showed multiple calcifications in the aorta and visceral arteries, as well as calcifications in the pancreaticoduodenal arcade (Figure 1). In addition to the calcifications, the arterial phase of the CT showed: (1) a focal narrowing in the proximal celiac trunk with a “hooked” appearance characteristic of a MAL; and (2) arterial supply from the SMA to the common hepatic artery via the GDA, as well as a dorsal pancreatic artery (Figure 2A and B).
Exploratory laparotomy showed no contraindication to resection. Para-aortic lymph node biopsy showed no metastasis. Peroperative ultrasound showed a large pancreaticoduodenal arcade and a large dorsal pancreatic artery. However, the preoperative CT scan had underestimated the local extension because evidence of tumor abutment on the mesenteric vein existed. We performed a MAL division using a lateral approach allowing for a progressive division of the right diaphragmatic crus on the right side of the abdominal aorta, and the right side and the upper edge of the CA was progressively freed of all dense fibrous tissue. An additional GDA clamping test with Doppler ultrasound monitoring showed unsatisfactory restoration of the liver blood flow through the CA. Thus, considering the tumor as “borderline” resectable, revascularization of the hepatic artery and PD were both postponed. The postoperative course was uneventful. The in-hospital stay was 7 d.
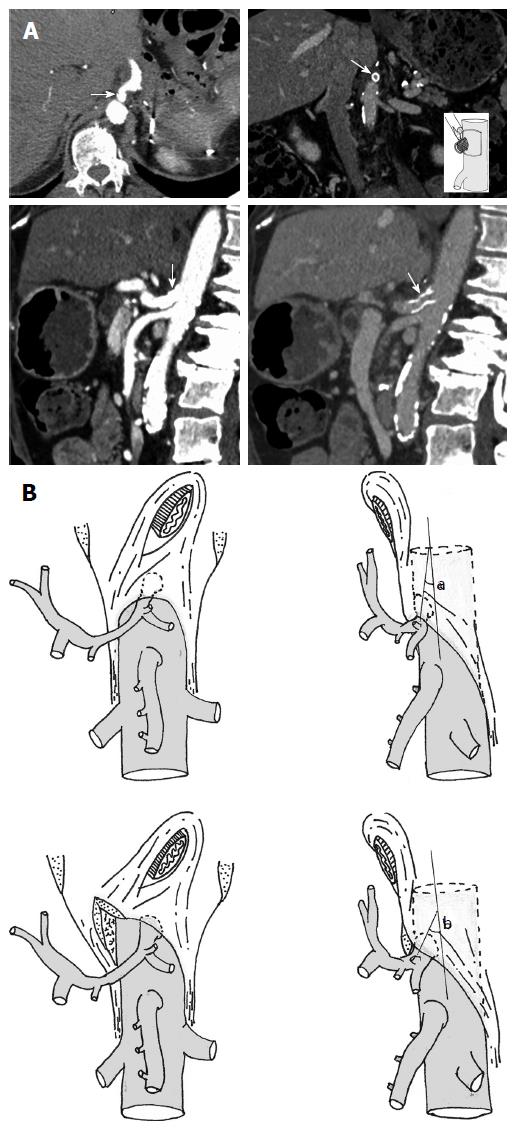
The patient received 4 cycles of neoadjuvant FOLFIRINOX before imaging reassessment and endovascular management. Endovascular revascularization was performed 45 d after the first surgical step, during the interval between 2 cycles of chemotherapy. A CT scan showed modification of the CA/aorta “angle” after MAL release, which allowed for the possibility of a much easier stenting. Selective arteriography of the CA showed a short and significant remaining proximal stenosis of the CA. A careful crossing of the stenosis allowed angioplasty followed by stenting (Figure 3A and B). Subsequently, the CA blood flow was restored and the duodenopancreatic arterial supply disappeared.
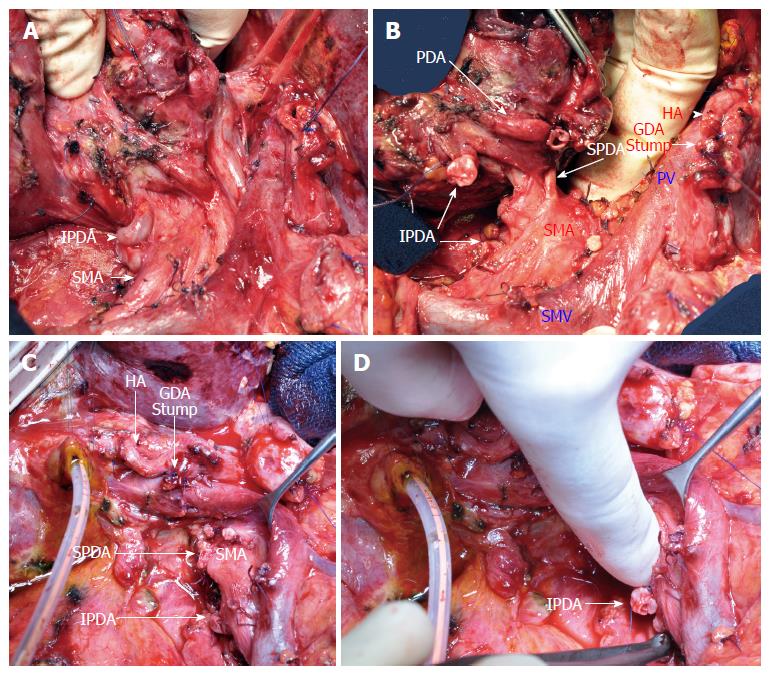
After 6 cycles of chemotherapy with a normalization of CA19-9 and an objective response on CT, a PD was performed without vein resection (Figure 4). The hepatic arterial inflow was preserved after GDA and dorsal pancreatic artery clamping and division. The divided common bile duct was well vascularized.
The standardized pathological examination of the specimen showed a 20 mm yp T3N1 poorly differentiated pancreatic adenocarcinoma with perineural involvement (6/10 positive nodes; lymph node ratio: 0.6). The resection was R0 as the inked margins were all negative; SMA, venous and posterior inked margins were free of tumor with a more than 1 mm clearance.
The postoperative course was uneventful. The patient was discharged on day 15 after equilibration of the diabetes. Adjuvant chemotherapy was performed for 6 mo. After 18 mo of follow-up, the patient was well and recurrence-free.
In recent years, mortality after PD has continuously decreased and today is less than 3% in high-volume centers[3,23]. However, ischemic complications are underestimated[1], and preoperative unrecognized visceral artery stenosis (e.g., CA, SMA) may severely alter the early postoperative outcomes of PD with a high mortality rate[1-9]. In cases with CA stenosis, PD results in an interruption of the alternate pathway for blood supply to the liver. After biliary anastomosis, hepaticojejunostomy leakage, liver abscesses with sepsis, liver insufficiency and subsequent multiple organ failure represent a major cause of death[3-5,24,25].
CA stenosis is detected during the preoperative staging of pancreatic cancer in 4%-11% of patients scheduled for PD[1,2,9,10,18]. The majority of these detected stenoses are asymptomatic before the diagnosis of the tumor. Despite an increased number of aged patients undergoing PD in recent years, atherosclerotic intrinsic stenosis is rare; Gaujoux et al[1]. reported a rate of 0.04% in a large series of 545 patients undergoing PD [2/57 (3.5%) visceral artery stenosis]. Instead, MAL is the major cause of CA stenosis. The liver blood supply is provided mostly by the pancreaticoduodenal arcades (95%) and the dorsal pancreatic artery (77%)[10-12,26]. Most cases are asymptomatic (10, 16, 19 and 20) and hemodynamically significant stenosis during the GDA clamping test has been reported to be present in about 40% of the cases, which represents 5% of the patients submitted to PD[1,13].
Atherosclerotic stenosis should be treated preoperatively by endovascular stenting. In contrast, for cases with MAL, a ligament release is the first mandatory procedure and a lateral approach is less risky than a medial approach, which may result in an arterial injury: MAL-induced CA stenosis is reported to be successfully treated by ligament release in nearly 90% of cases[1,2,9,20]. In the recent series reported by Gaujoux et al[1], 20 out of 23 MAL releases were successful with no postoperative mortality[1]. If there is not restitution of flow in the hepatic artery after MAL release, then a vascular reconstruction prior to division of the GDA should be considered before proceeding with the resection. Indeed, preoperative MAL stenting is technically challenging and proves mostly ineffective by the default of expanding and restenosis. In the current report, stenting was easier after MAL release and this strategy should be considered as an alternative to vascular bypass, avoiding the risk of postoperative thrombosis[13,16-21]. Secondary stenting should proceed with cautious regard for the potential risk of injury during the procedure due to the fragility of the artery after ligament release. A successful laparoscopic approach for a MAL division has been reported in symptomatic patients with MAL syndrome but without pancreatic tumor. In cases of CA stenosis detected by a multidetector CT scan before PD of a clearly resectable pancreatic adenocarcinoma, the following strategy should be considered as an option: (1) in cases with a hemodynamically significant stenosis, as assessed by GDA clamping and Doppler ultrasound, perform a first-step, including MAL release, with abdominal exploration and a para-aortic lymph node biopsy (currently recommended in order to avoid futile resection)[27,28]; (2) in cases involving a failure of MAL release to restore the liver blood inflow, perform secondary cautious endovascular stenting of the CA in order to avoid the risk of postpancreatectomy arterial reconstruction thrombosis and postpone PD. A laparoscopic or robotic approach should be considered for the first step[29].
In conclusion, CA stenosis is usually due to a previously asymptomatic MAL which induces a major risk of post-PD complications due to liver and biliary ischemia if unrecognized before the resection. The GDA clamping test is mandatory in order to detect hemodynamically significant stenosis. MAL release is usually efficient to restore an adequate liver blood inflow. Postponed PD after inefficient MAL release, followed by secondary stenting, should be considered as a two-stage option that avoids hepatic artery bypass and the postoperative risk of thrombosis of the arterial reconstruction.
We would like to thank Editage (http://www.editage.com) for English language editing.
Failure in median arcuate ligament (MAL) release during pancreaticoduodenectomy (PD) for pancreatic adenocarcinoma
Celiac axis (CA) stenosis due to MAL in a patient with pancreatic adenocarcinoma.
Postoperative computed tomography scan showed modification of the CA/aorta “angle” after MAL release, which allowed for the possibility of a much easier stenting.
Postponed PD and secondary stenting of the CA as an alternative to hepatic artery reconstruction
MAL release is usually efficient (90%) to restore an adequate liver blood inflow and prevent ischemic complications; in case of inefficient release hepatic artery reconstruction is usually indicated.
Preoperative MAL stenting is technically challenging and proves mostly ineffective by the default of expanding and restenosis; in the current report, stenting was easier after MAL release and this strategy should be considered as an alternative to vascular bypass, avoiding the risk of postoperative thrombosis; secondary stenting should proceed with cautious regarding the potential risk of injury during the procedure due to the fragility of the artery after ligament release.
Great work on a theme really important in our diary practice.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kelemen D, Silva CR S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Gaujoux S, Sauvanet A, Vullierme MP, Cortes A, Dokmak S, Sibert A, Vilgrain V, Belghiti J. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Columbo JA, Trus T, Nolan B, Goodney P, Rzucidlo E, Powell R, Walsh D, Stone D. Contemporary management of median arcuate ligament syndrome provides early symptom improvement. J Vasc Surg. 2015;62:151-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 896] [Cited by in F6Publishing: 922] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 4. | Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, Hruban RH, Ord SE, Sauter PK, Coleman J. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248-257; discussion 257-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1357] [Cited by in F6Publishing: 1359] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 5. | Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788-797; discussion 797-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 613] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 6. | Fortner JG, Watson RC. Median arcuate ligament obstruction of celiac axis and pancreatic cancer. Ann Surg. 1981;194:698-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Bull DA, Hunter GC, Crabtree TG, Bernhard VM, Putnam CW. Hepatic ischemia, caused by celiac axis compression, complicating pancreaticoduodenectomy. Ann Surg. 1993;217:244-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Thompson NW, Eckhauser FE, Talpos G, Cho KJ. Pancreaticoduodenectomy and celiac occlusive disease. Ann Surg. 1981;193:399-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Ouassi M, Verhelst R, Astarci P, El Khoury G, Hubert C, van Beers BE, Annet L, Goffette P, Noirhomme P, Gigot JF. Celiac artery occlusive disease: a rare but potentially critical condition in patients undergoing pancreaticoduodenectomy. Hepatogastroenterology. 2011;58:1377-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25:1177-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Blomley MJ, Albrecht T, Williamson RC, Allison DJ. Three-dimensional spiral CT angiography in pancreatic surgical planning using non-tailored protocols: comparison with conventional angiography. Br J Radiol. 1998;71:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Song SY, Chung JW, Kwon JW, Joh JH, Shin SJ, Kim HB, Park JH. Collateral pathways in patients with celiac axis stenosis: angiographic-spiral CT correlation. Radiographics. 2002;22:881-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Farma JM, Hoffman JP. Nonneoplastic celiac axis occlusion in patients undergoing pancreaticoduodenectomy. Am J Surg. 2007;193:341-344; discussion 344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Winter JM, Cameron JL, Yeo CJ, Alao B, Lillemoe KD, Campbell KA, Schulick RD. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2007;204:1029-1036; discussion 1037-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Park CM, Chung JW, Kim HB, Shin SJ, Park JH. Celiac axis stenosis: incidence and etiologies in asymptomatic individuals. Korean J Radiol. 2001;2:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Kurosaki I, Hatakeyama K, Nihei KE, Oyamatsu M. Celiac axis stenosis in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2004;11:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Miyata M, Takao T, Okuda A, Sasako Y, Sunada S. Pancreatoduodenectomy for periampullary cancer associated with celiac occlusion: a case report. Surgery. 1988;103:261-263. [PubMed] [Cited in This Article: ] |
| 18. | Okamoto H, Suminaga Y, Toyama N, Konishi F, Kawahito H. Autogenous vein graft from iliac artery to splenic artery for celiac occlusion in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2003;10:109-112. [PubMed] [Cited in This Article: ] |
| 19. | Murakami Y, Uemura K, Yokoyama Y, Sasaki M, Morifuji M, Hayashidani Y, Sudo T, Sueda T. Celiac axis occlusion with replaced common hepatic artery and pancreatoduodenectomy. J Gastrointest Surg. 2004;8:520-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Portolani N, Tiberio GA, Coniglio A, Baiocchi G, Vettoretto N, Giulini SM. Emergency celiac revascularization for supramesocolic ischemia during pancreaticoduodenectomy: report of a case. Surg Today. 2004;34:616-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Jimenez JC, Harlander-Locke M, Dutson EP. Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg. 2012;56:869-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology, Pancreatic adenocarcinoma, Version I, 2016. [accessed 2016 Apr 1]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/. [Cited in This Article: ] |
| 23. | Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, Howard TJ, Pitt HA, Lillemoe KD. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145:634-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 334] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 24. | Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 307] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 25. | Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310-1314; discussion 1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 434] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 26. | Trede M. Vascular problems and techniques associated with pancreatectomy and regional pancreatectomy. In: Trede M, Carter DC, editors. Surgery of the Pancreas. Edinburgh: Churchill Livingstone, 1993: 465- 476. . [Cited in This Article: ] |
| 27. | Schwarz L, Lupinacci RM, Svrcek M, Lesurtel M, Bubenheim M, Vuarnesson H, Balladur P, Paye F. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg. 2014;101:530-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Paiella S, Sandini M, Gianotti L, Butturini G, Salvia R, Bassi C. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2016;42:616-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 29. | Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. Median Arcuate Ligament Syndrome-Review of This Rare Disease. JAMA Surg. 2016;151:471-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |