Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8271
Peer-review started: April 27, 2016
First decision: June 20, 2016
Revised: July 18, 2016
Accepted: August 5, 2016
Article in press: August 5, 2016
Published online: October 7, 2016
Chronic hepatitis B virus (HBV) infected patients have an almost 100-fold increased risk to develop hepatocellular carcinoma (HCC). HCC is the fifth most common and third most deadly cancer worldwide. Up to 50% of newly diagnosed HCC cases are attributed to HBV infection. Early detection improves survival and can be achieved through regular screening. Six-monthly abdominal ultrasound, either alone or in combination with alpha-fetoprotein serum levels, has been widely endorsed for this purpose. Both techniques however yield limited diagnostic accuracy, which is not improved when they are combined. Alternative circulating or histological markers to predict or diagnose HCC are therefore urgently needed. Recent advances in systems biology technologies have enabled the identification of several new putative circulating biomarkers. Although results from studies assessing combinations of these biomarkers are promising, evidence for their clinical utility remains low. In addition, most of the studies conducted so far show limitations in design. Attention must be paid for instance to different ethnicities and different etiologies when studying biomarkers for hepatocellular carcinoma. This review provides an overview on the current understandings and recent progress in the field of diagnostic and predictive circulating biomarkers for hepatocellular carcinoma in chronically infected HBV patients and discusses the future prospects.
Core tip: Regular screening for hepatocellular carcinoma (HCC) in patients at risk improves their survival rates. Currently available screening methods include abdominal ultrasound and alpha-fetoprotein serum levels, but both methods lack diagnostic accuracy. Recent technological advances have enabled the identification of new predictive and diagnostic hepatitis B virus (HBV)-associated HCC biomarkers. Nevertheless, most of the studies conducted so far show design limitations. This review provides an overview on the current understanding and future prospects of circulating predictive and diagnostic biomarkers for HBV-associated HCC.
- Citation: Van Hees S, Michielsen P, Vanwolleghem T. Circulating predictive and diagnostic biomarkers for hepatitis B virus-associated hepatocellular carcinoma. World J Gastroenterol 2016; 22(37): 8271-8282
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8271.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8271
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and ranks third as cancer-related death cause due to a 5-year survival of only 15%[1]. Moreover, at a time of decreasing overall cancer-related deaths due to an immense progress in cancer diagnostics and treatment options, mortality from hepatocellular carcinoma is increasing[1,2].
Chronic hepatitis B virus (HBV) infection is a major risk factor for HCC development. Prospective cohort studies have revealed an up to 100-fold increased risk for HCC in chronically infected HBV patients[3]. Up to 50% of newly diagnosed HCC cases are attributed to HBV infection, due to both direct and indirect oncogenic effects of the virus[2,4-7]. Integration of HBV DNA in the human genome may result in genomic instability, while inflammation-related oxidative stress, caused by immunological responses, may contribute indirectly to HCC development[6-9].
Four clinical phases can be distinguished during the natural course of a HBV infection: an immune-tolerance phase, an immune active phase, an inactive carrier phase and a hepatitis B e antigen (HBeAg) negative phase[2,10]. Patients in the immune active phase and the HBeAg negative phase are at increased risk for progression towards fibrosis and ultimately the development of cirrhosis, which is a major risk factor for HCC[11,12].
Several years to decades are needed for HCC to develop in a HBV infected liver[13]. Early diagnosis of HCC in HBV patients is challenging but is proven to result in an improved long-term survival due to an increased chance to detect tumors at a resectable stage[14-19].
Importantly, also a significant number of HBV patients develop HCC in a non-cirrhotic liver[20]. Current guidelines therefore advise 6-monthly abdominal ultrasound (US) surveillance for HCC in advanced fibrosis or cirrhotic HBV patients and in non-cirrhotic patients depending on ethnic background and age[21-25]. The technique, however, faces a disappointing 63% sensitivity to detect HCC and is hampered by inter- and intra-observer variability[20]. Finding biomarkers to better predict or diagnose HCC therefore remains an important clinical and research priority.
Serum alpha-fetoprotein (AFP) levels are widely used for HCC screening and diagnostics, but the clinical utility to rule out or detect HCC is still a matter of debate. The protein lacks sensitivity and specificity to detect HCC. The recent improvement of systems biology techniques, such as proteomics and genomics, has enabled the identification of several new putative biomarkers[26,27]. This review provides an overview of diagnostic and predictive serum biomarkers for HBV-associated hepatocellular carcinoma and discusses future prospects.
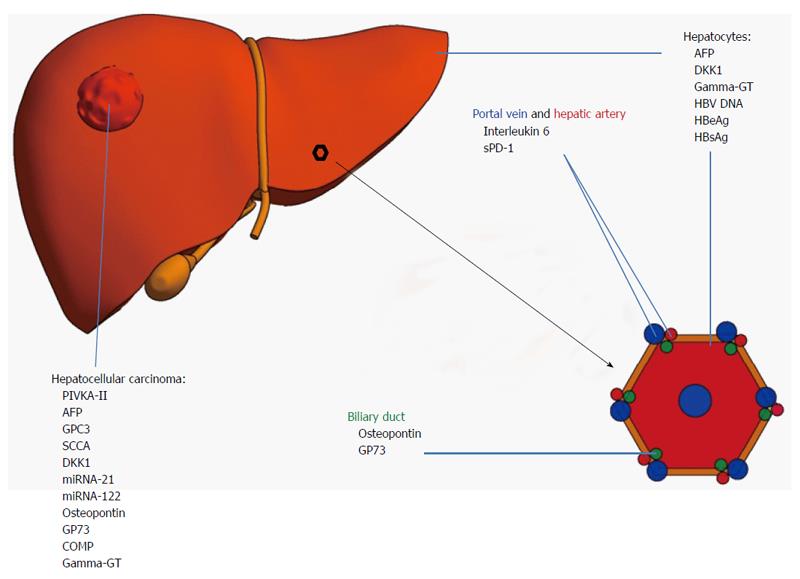
An overview of the discussed diagnostic circulating biomarkers with their respective sensitivities and specificities to detect HCC is displayed in Table 1. An overview of their cellular origin is displayed in Figure 1.
| Marker | Cut-off1 | Sensitivity | Specificity | Detection method (most reliable) | Ref. |
| AFP | 7.7-112.0 ng/mL | 25%-90% | 87%-97% | ELISA | [36,73] |
| AFP-l3 | 3%-20% | 36%-96% | 89%-94% | Liquid-Phase Binding Assay | [46,47,52] |
| DCP | 40-150 mAU/mL | 44%-91% | 68%-99% | Electrochemiluminescence immunoassay | [36,53] |
| Osteopontin | 9.3-642.5 ng/mL | 73%-97% | 55%-100% | ELISA | [65] |
| GP73 | 78-150 ng/mL | 68%-95% | 9%-97% | Immunoblotting, Western Blotting or ELISA2 | [36,71,72] |
| GPC-3 | 2-300 ng/mL | 36%-100% | 40%-100% | ELISA | [36,78] |
| SCCA | 0.12-3.80 ng/mL | 42%-80% | 50%-88% | ELISA | [36,81] |
| DKK1 | 1.01-2.15 ng/mL | 69%-91% | 62%-91% | ELISA | [86-88] |
| miRNA-21 | NA | 84%-90% | 71%-92% | qRT-PCR | [83] |
| miRNA-122 | NA | 70%-82% | 69%-84% | qRT-PCR | [83] |
Alpha-fetoprotein is an oncofetal protein produced by the fetal yolk sac and liver[28]. The protein, like albumin, binds exogenous as well as endogenous substances in blood[29]. Physiologically elevated AFP levels are found in pregnant women and newborns, but decrease quickly after birth. Upregulation of AFP later on in life has been associated with various pathological conditions such as acute hepatitis, endodermal sinus tumors and HCC[30-32].
Alpha-fetoprotein was discovered in the late 1950s and has been of interest for the monitoring of HCC development in viral hepatitis patients since the early 1970s[33-35]. For a long time, AFP has been widely used together with abdominal US in routine HCC screening. Nevertheless, the most recent European and American guidelines do not endorse this practice anymore since its diagnostic accuracy is low[21-23]. The protein, most often detected by enzyme-like immunosorbent assays (ELISA), indeed faces a lack of sensitivity and specificity to detect early stage HCC in HBV patients[36]. Only 70% of all HCC’s are characterized by markedly elevated AFP levels at the time of diagnosis[37-41]. Large HBV cohort studies showed a maximal sensitivity for AFP of about 75% to detect HCC at optimal cut-off levels[32,41-45].
AFP is a glycoprotein of which three glycoforms exist: AFP-l1, AFP-l2 and AFP-l3. They are all characterized by an increased binding affinity for Lens culinaris agglutinin. AFP-l3, which shows the highest binding affinity is of particular interest as a biomarker for hepatocellular carcinoma. This glycoform is secreted by malignant HCC cells even at early tumor stages and in the absence of elevated AFP levels and can be detected using liquid-phase binding assays[46,47]. In addition, the fraction of AFP-l3 to total AFP in the serum correlates with the degree of malignancy[48]. Over 15 studies have addressed the clinical potential of AFP-l3 so far with sensitivity and specificity ranging from 21% to 84% and from 89% to 94% respectively[48-52]. However these studies assessing the clinical potential of AFP-l3 use different cut-off levels, test methods and patient numbers, resulting in a wide range of detected sensitivity. A study from 2009 measuring the fraction of AFP-l3 to total AFP using an automated immunologic analyzer and a cutoff of 10% AFP-l3 in 419 HCC patients and 417 cirrhotic controls, found a sensitivity of 42% to detect HCC[53]. AFP-l3 fractions were measured using Western blotting in another study, involving 388 HCC patients and 212 controls with a cutoff of 15% AFP-l3 to total AFP, resulting in a sensitivity of 21%[54]. In order to unequivocally demonstrate the superiority of AFP-l3 to AFP, large cohort studies using the same cutoff and detection method are needed. Recently AFP-l3 was suggested to be especially useful in the diagnosis of HCC in absence of elevated AFP levels, but further validation is needed[55].
Des-gamma carboxy prothrombin (DCP) is a non-carboxylated form of prothrombin, also known as protein induced by vitamin K absence (PIVKA-II). Carboxylation takes place in the hepatocytes before the protein is released into the circulation. Release of the non-carboxylated form has been associated with vitamin K deficiency and presence of HCC[56]. Elevated DCP levels, preferably measured using electrochemoluminiscence assays, were found in sera of HCC patients, suggesting proper DCP synthesis in hepatoma cells[36,57,58]. DCP has been investigated as a potential HCC diagnostic biomarker in several studies, showing a comparable to slightly higher diagnostic performance compared to AFP[59-63].
Osteopontin (OPN) is a glycoprotein that constitutes a major part of the extracellular matrix of bones and teeth. In addition, low levels of the protein are being secreted by biliary epithelial cells. OPN is involved in developmental as well as immunological, tumorigenic and bone homeostatic processes[64]. Overexpression of the protein, detected using ELISA assays, was found in a wide range of tumor types including pancreas cancer, multiple myeloma and HCC[64-66]. Seven retrospective cohort studies have investigated the diagnostic potential of OPN for HCC. So far, OPN does not outperform AFP as a diagnostic marker[65].
Golgi protein-73 (GP73) is a transmembrane protein physiologically located on the Golgi membrane of epithelial cells in different tissues, including the biliary tract[67]. Its function remains largely unknown. Liver damage, caused by viral as well as non-viral agents leads to GP73 upregulation[68,69]. Increasing GP73 serum levels are associated with advanced fibrosis stages in HBV patients[70,71]. A recent meta-analysis showed that GP73’s diagnostic accuracy for HCC outperforms that of AFP[72,73]. The protein can be detected using either ELISA assays, immunoblotting or Western blot. Previous studies have shown a comparable efficacy for all three methods[36,71].
Glypican-3 (GPC3) is a member of the heparan sulfate proteoglycans. It is an oncofetal antigen involved in embryonal morphogenesis[74]. Significant expression in human adults can occur in different tissues including breast and liver and indicates ongoing pathological, mostly carcinogenic processes[75,76]. GPC3 has been proposed as a novel serum marker for HCC[75]. The protein promotes HCC tumor growth through stimulation of the Wnt signaling pathway[77]. A recent meta-analysis showed an acceptable accuracy of the protein to detect HCC with a mean pooled sensitivity and specificity of 56% and 89% respectively[78]. The protein is preferably detected using ELISA assays[36].
Squamous cell carcinoma antigen (SCCA) is a serine protease inhibitor, physiologically located in squamous epithelial cells. It is also expressed by neoplastic epithelial cells, e.g., neoplastic liver cells in which it promotes tumor growth through inhibition of apoptosis[79,80]. Increased serum levels have been detected using ELISA assays in HCC patients[36]. The protein’s diagnostic accuracy for HCC has been investigated in over 12 studies and turned out to be moderate with a pooled sensitivity and specificity of 59.0% and 76.0% respectively. Nevertheless some design limitations of these studies such as a small sample size need to be taken into account[81].
Dickkopf-1 protein (DKK1) is a glycoprotein secreted by human hepatocytes. Upregulation of DKK1 expression takes place in a wide variety of cancers including prostate cancer, multiple myeloma and hepatocellular carcinoma[82-84]. Overexpression of the protein is detected in tissue as well as serum from hepatocellular carcinoma patients. Although the protein is suggested to be an inhibitor of the Wnt/β-catenin signaling pathway, its exact functions have not been fully elucidated[82,85]. A meta-analysis showed an acceptable diagnostic accuracy of DKK1, comparable to AFP, to detect HCC with a pooled sensitivity and specificity of 65% and 94% respectively[86-88]. Detection of the protein in serum is performed using ELISA assays[87].
MicroRNA’s (miRNA) are small non-coding RNA’s regulating gene expression by binding to messenger-RNA (mRNA)[89]. During recent years, circulating miRNAs have gained increasing attention for the early diagnosis and screening of hepatocellular carcinoma[90]. So far, two miRNAs, miRNA-21 and miRNA-122 show particularly high potential in HCC diagnostics[91]. miRNA-122 is a liver specific miRNA, whereas miRNA-21 is produced by different tissues including the colon, liver and heart in which it is involved in respectively tumor growth and cardiac disease development[92-94]. miRNA-21 inhibits tumor suppression by inhibiting tumor suppressor pathway activating phosphatases (e.g., ATK and MAPK), whereas miRNA-122 inhibits tumor growth by acting as a tumor suppressor gene[93,95-97]. A direct correlation was observed between increasing miRNA-21 levels and increased cell proliferation[98]. In addition, high circulating miRNA-21 levels were found to be correlated with more differentiated and progressive hepatocellular carcinoma thus indicating a bad prognosis[94]. Serum miRNA-122 levels correlate inversely with the severity of liver fibrosis[99]. The antitumor properties of miRNA-122 have been successfully applied in a preclinical model to prevent HCC development[96]. The diagnostic accuracy of miRNA-21 slightly outperforms that of miRNA-122 with a pooled sensitivity and specificity of 87% and 80% respectively for miRNA-21 vs 68% and 73% for miRNA-122[91].
Based on systems biology approaches, more markers with diagnostic potential in HCC screening settings have recently been identified, including fucosylated fetuin A, inter-alpha-trypsin inhibitor H4, clusterin, endoglin, soluble Axl, latent TGF-bèta binding-protein 2 as well as peroxireduxin 1, 2 and 3. The evidence for clinical utility of these markers remains low due to a lack of sufficiently large cohort studies[100-108].
In addition, several studies have been published on circulating tumor cells for HCC. However, most of published studies focus on prognosis after HCC diagnosis and prediction of disease progression rather than on the diagnosis of HCC[109-111].
Three strategies can be applied when assessing the long-term risk for HCC. Firstly, clinical risk scores, e.g., REACH-B and PAGE-B, can be calculated based on viral and host-related (e.g., age and gender) clinical parameters. Most of these models have however been developed in Asian populations and lack validation in non-Asian populations[112,113]. HBeAg positivity and HBV DNA levels above 1 million copies/mL are associated with a 4- and 11-fold increased HCC-risk during 8 and 11 years of follow-up respectively[114-116]. Hepatitis B surface antigen (HBsAg) levels above 1000 IU/mL are accompanied with an up to 6.5-fold increased HCC risk in men and an up to 11-fold increased risk in women within 15 years[117]. HBsAg levels are suggested to be especially useful in case of low HBV DNA levels[118,119].
Secondly, genome-wide association studies have enabled the linkage of genetic variants to specific disease outcomes. Single nucleotide polymorphisms (SNPs) in a wide range of genes, including the Interleukin-21 and the CRP-gene, have been associated with an increased susceptibility for HCC over a variable time course[120-124]. Increasing evidence indicates that SNP’s in the STAT4, MDM2 and HFE gene, determined on whole blood, are germline risk factors for HCC[125,126]. On the other hand, also somatically acquired mutations, e.g., in the TP53 gene, have been associated with an increased risk for HCC[127]. All together these findings are strongly suggestive for interindividual differences in the genetic predisposition for HCC development, a predisposition that can be boosted by additional somatic mutations.
Thirdly, circulating biomolecules would be ideal as a non-invasive, predictive biomarker for HCC. An overview on the discussed predictive biomarkers including their respective increase in HCC risk is displayed in Table 2. The clinical utility of Gamma-Glutamyl Transferase (Gamma-GT) Iso-enzyme II was first evaluated as a predictive HCC marker in 1992. Patients showing persistently elevated levels of Gamma-GT Iso-enzyme II at presentation had a 86.7% risk to develop HCC within 10 years’ time[128]. Gamma-GT levels above 41 U/L and AFP-levels > 5 ng/mL have later been associated with an 8-fold increased risk for HCC in a large HBV cohort followed up for 6 years[129]. The usefulness of AFP-levels for HCC prediction has, however, been assessed in several other studies with contradictory results[128-130].
| Marker | Cut-off | Increased risk for HCC | Control group1 | Follow-up time | Ref. | |
| Viral | HBeAg | Positive | 4-fold | HBeAg negative HBV patients | 8 yr | [114] |
| HBV DNA | > 1 million copies/mL | 11-fold | HBV patients with HBV DNA < 300 copies/mL | 11 yr | [115] | |
| HBsAg | > 1000 IU/mL | 3-fold | HBV patients with HBsAg 5-9 IU/mL | 14.7 yr | [117] | |
| Host | Gamma-GT Iso-enzyme II | Positive | 86-fold | GGT Iso-enzyme II negative HBV patients | 10 yr | [128] |
| Gamma-GT | > 41 U/L | 8-fold | HBV patients with Gamma-GT ≥ 41 U/L | 5.9 yr | [129] | |
| AFP | > 5 ng/mL | 8-fold | HBV patients with AFP ≤ 5 ng/mL | 5.9 yr | [129] | |
| COMP | Positive | 3-fold | COMP negative HBV and HCV patients | 8 yr | [134] | |
| IL-6 | > 7 pg/mL | 3-fold | HBV patients with IL-6 < 7 pg/mL | 7.25 yr | [135] | |
| sPD-1 | > 637.6 pg/mL | 2-fold | HBV patients with sPD-1 < 117.3 pg/mL | 20 yr | [138] |
Cartilage oligomeric matrix protein (COMP) is an extracellular matrix protein involved in tissue genesis and remodeling[131]. The protein is released into the circulation upon cartilage damage[132]. Overexpression of the protein in serum from HCC patients suggested that serum COMP levels reflected an individual’s fibrosis stage and subsequent risk for HCC[133]. A recent study in Greece supports this hypothesis: COMP positivity (> 15 U/L) was associated with a 3-fold increased HCC risk during a median follow-up of 8 years[132,134].
In a study of 27 serum cytokines and growth factors, interleukin-6 (IL-6) levels were found to predict cancer development within a timeframe of 8 to 11 years with moderate accuracy[135]. Levels above 7 pg/mL were associated with a 3-fold increased HCC risk. As IL-6 induces C-reactive protein (CRP), the potential value of CRP in HCC risk prediction was assessed, but turned out to be disappointing[136,137]. Recently, soluble programmed death-1 (sPD-1), a soluble form of the membrane-bound programmed death 1 on T cells with a largely unknown function was put forward as a marker[138,139]. sPD-1 levels above 637.6 pg/mL at baseline reflected a 2-fold increased risk to develop HCC during a median follow-up time of 20 years, when compared to sPD-1 levels below 117.3 pg/mL[138].
Despite its disappointing sensitivity and specificity, AFP still remains the most widely used serum HCC biomarker. Some newly discovered circulating biomarkers, e.g., AFP-l3, DCP and microRNA’s show promising potential for implementation in clinical practice. However, only GP73 strongly outperforms AFP in terms of diagnostic accuracy. Large variations in sensitivity and specificity are noticed between different studies assessing the same biomarker (Table 1).
Due to the heterogeneity of HCC, one single biomarker with 100% sensitivity and specificity in all HCC cases will be hard to find. A more rational approach to increase the diagnostic accuracy might be the combination of different biomarkers[41,52,55,62,65,72,140-142]. The most recent APASL guidelines indeed recommend the combined use of AFP, AFP-l3 and DCP in HCC screening[22,142]. In favor of this approach, a meta-analysis showed that combined testing of GP73 and AFP increased the pooled sensitivity without decreasing the specificity to detect hepatocellular carcinoma. The pooled sensitivity and specificity were 87% and 85% respectively when biomarkers were combined, compared to 77% and 91% for GP73 and 62% and 84% for AFP when used alone[72].
All currently identified circulating biomarkers and their combinations definitely need more validation studies. Most of the biomarker discovery studies have been performed in cohorts of a few 100 patients. The majority of identified markers has so far not been subject of large, external validation studies[100-103,108]. Five subsequent steps are to be followed in cancer biomarker discovery. The first step is the implementation of preclinical exploratory studies. Step 2 is the development of a clinical assay. Step 3 involves retrospective studies, step 4 prospective studies and step 5 large, randomized controlled trials. Biomarkers identified in step 1 must pass all other steps before they can be termed validated biomarkers[143]. So far, only AFP has reached step 5[15].
In addition, the studies that have been conducted over the last decades are hampered by limitations in their study design. As an example the patient cohorts for HCC biomarker discovery studies are often heterogeneous regarding liver disease etiology and ethnicity. In their paper, da Costa et al[144] proved the need to validate biomarkers in different ethnic populations. They investigated the potential of osteopontin and latent Transforming Growth Factor beta binding-protein in HCC diagnosis in separate cohorts in Gambia, Korea, Thailand and France. The sensitivity and specificity of both markers differed (> 10%) among ethnicities. The onset of HCC occurs at a median age of 45 in sub-Saharan African people, whereas a mean age of 52 to 65 has been observed in the rest of the world[145,146]. In addition, HCC incidence varies among HBV and hepatitis C virus (HCV) patients, highlighting the importance of homogeneous patient cohorts. Future biomarker discovery and validation studies should therefore distinguish between different ethnicities and etiologies as this most probably explains the variation in sensitivities and specificities noticed between studies assessing the same biomarker (Table 1).
The inclusion of clinical parameters into biomarker scores could increase their performance. One study demonstrated that incorporation of age into combined models of biomarker testing significantly improved the diagnostic performance for HCC[140].
From a clinical point of view, however, predictive serum biomarkers would be preferred over diagnostic biomarkers to tailor HCC surveillance according to the individual needs. Proteomic approaches are encouraging, but also need a validation in larger cohorts[147,148]. Expression of Heat-shock Protein 27 was e.g., detected in 90% of sera from HCC patients and in 0% of sera from non-HCC patients, which seems promising[148]. Other groups have focused on genomics and have identified a gene signature in liver tissue of HCV infected patients predictive of HCC development[149,150]. It could be of interest to identify corresponding secretory biomarkers in blood.
Monitoring HCC development in chronic hepatitis B patients based on serum biomarkers remains challenging. During recent years, new predictive and diagnostic circulating biomarkers have been proposed. Combinations of these biomarkers show a higher potential for implementation in clinical practice, but large validation studies in homogeneous ethnic and etiological populations are urgently needed to unequivocally demonstrate their clinical utility.
The authors thank professor Benedicte Y De Winter for the critical revision and language editing of the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gao YT, Wan SS, Zhou XH S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12135] [Cited by in F6Publishing: 12698] [Article Influence: 1587.3] [Reference Citation Analysis (2)] |
| 2. | Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 663] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2183] [Cited by in F6Publishing: 2337] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 4. | Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris). 2010;58:273-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2881] [Cited by in F6Publishing: 2977] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 6. | Higgs MR, Chouteau P, Lerat H. ‘Liver let die’: oxidative DNA damage and hepatotropic viruses. J Gen Virol. 2014;95:991-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Sukowati CH, El-Khobar KE, Ie SI, Anfuso B, Muljono DH, Tiribelli C. Significance of hepatitis virus infection in the oncogenic initiation of hepatocellular carcinoma. World J Gastroenterol. 2016;22:1497-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 40] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Bonilla Guerrero R, Roberts LR. The role of hepatitis B virus integrations in the pathogenesis of human hepatocellular carcinoma. J Hepatol. 2005;42:760-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Michielsen P, Ho E. Viral hepatitis B and hepatocellular carcinoma. Acta Gastroenterol Belg. 2011;74:4-8. [PubMed] [Cited in This Article: ] |
| 10. | Vanwolleghem T, Hou J, van Oord G, Andeweg AC, Osterhaus AD, Pas SD, Janssen HL, Boonstra A. Re-evaluation of hepatitis B virus clinical phases by systems biology identifies unappreciated roles for the innate immune response and B cells. Hepatology. 2015;62:87-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Vlachogiannakos J, Papatheodoridis GV. HBV: Do I treat my immunotolerant patients? Liver Int. 2016;36 Suppl 1:93-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49:S56-S60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 14. | McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, Williams J. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 264] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 751] [Cited by in F6Publishing: 848] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 16. | Miller ZA, Lee KS. Screening for hepatocellular carcinoma in high-risk populations. Clin Imaging. 2015;40:311-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KM, Klümpen HJ, Jansen PL, IJzermans JN, van Oijen MG, Siersema PD. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol. 2015;63:1156-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Velázquez RF, Rodríguez M, Navascués CA, Linares A, Pérez R, Sotorríos NG, Martínez I, Rodrigo L. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4338] [Article Influence: 361.5] [Reference Citation Analysis (2)] |
| 22. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 732] [Cited by in F6Publishing: 813] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 23. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 24. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 25. | Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Vlaanderen J, Moore LE, Smith MT, Lan Q, Zhang L, Skibola CF, Rothman N, Vermeulen R. Application of OMICS technologies in occupational and environmental health research; current status and projections. Occup Environ Med. 2010;67:136-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Pesce F, Pathan S, Schena FP. From -omics to personalized medicine in nephrology: integration is the key. Nephrol Dial Transplant. 2013;28:24-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Seregni E, Botti C, Bombardieri E. Biochemical characteristics and clinical applications of alpha-fetoprotein isoforms. Anticancer Res. 1995;15:1491-1499. [PubMed] [Cited in This Article: ] |
| 29. | Gabant P, Forrester L, Nichols J, Van Reeth T, De Mees C, Pajack B, Watt A, Smitz J, Alexandre H, Szpirer C. Alpha-fetoprotein, the major fetal serum protein, is not essential for embryonic development but is required for female fertility. Proc Natl Acad Sci USA. 2002;99:12865-12870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Seo SI, Kim SS, Choi BY, Lee SH, Kim SJ, Park HW, Kim HS, Shin WG, Kim KH, Lee JH. Clinical significance of elevated serum alpha-fetoprotein (AFP) level in acute viral hepatitis A (AHA). Hepatogastroenterology. 2013;60:1592-1596. [PubMed] [Cited in This Article: ] |
| 31. | Guo YL, Zhang YL, Zhu JQ. Primary yolk sac tumor of the retroperitoneum: A case report and review of the literature. Oncol Lett. 2014;8:556-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Yao M, Zhao J, Lu F. Alpha-fetoprotein still is a valuable diagnostic and prognosis predicting biomarker in hepatitis B virus infection-related hepatocellular carcinoma. Oncotarget. 2016;7:3702-3708. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 364] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Tonami N, Aburano T, Hisada K. Comparison of alpha1 fetoprotein radioimmunoassay method and liver scanning for detecting primary hepatic cell carcinoma. Cancer. 1975;36:466-470. [PubMed] [Cited in This Article: ] |
| 35. | Akhmeteli MA, Linnik AB, Cernov KS. Hepatocarcinogenesis and the appearance of serum alpha-fetoprotein in mice treated with extracts of barley grain infected with Fusarium sporotrichioides. Bull World Health Organ. 1972;47:663-664. [PubMed] [Cited in This Article: ] |
| 36. | Waidely E, Al-Yuobi AR, Bashammakh AS, El-Shahawi MS, Leblanc RM. Serum protein biomarkers relevant to hepatocellular carcinoma and their detection. Analyst. 2016;141:36-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9:110-115. [PubMed] [Cited in This Article: ] |
| 38. | Maringhini A, Cottone M, Sciarrino E, Marcenó MP, La Seta F, Fusco G, Rinaldi F, Pagliaro L. Ultrasonography and alpha-fetoprotein in diagnosis of hepatocellular carcinoma in cirrhosis. Dig Dis Sci. 1988;33:47-51. [PubMed] [Cited in This Article: ] |
| 39. | He X, Wang Y, Zhang W, Li H, Luo R, Zhou Y, Liao CL, Huang H, Lv X, Xie Z. Screening differential expression of serum proteins in AFP-negative HBV-related hepatocellular carcinoma using iTRAQ -MALDI-MS/MS. Neoplasma. 2014;61:17-26. [PubMed] [Cited in This Article: ] |
| 40. | Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early recognition of hepatocellular carcinoma based on altered profiles of alpha-fetoprotein. N Engl J Med. 1993;328:1802-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 340] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 41. | Song P, Feng X, Inagaki Y, Song T, Zhang K, Wang Z, Zheng S, Ma K, Li Q, Kong D. Clinical utility of simultaneous measurement of alpha-fetoprotein and des-γ-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: A multi-center case-controlled study of 1,153 subjects. Biosci Trends. 2014;8:266-273. [PubMed] [Cited in This Article: ] |
| 42. | Kim GA, Seock CH, Park JW, An J, Lee KS, Yang JE, Lim YS, Kim KM, Shim JH, Lee D. Reappraisal of serum alpha-foetoprotein as a surveillance test for hepatocellular carcinoma during entecavir treatment. Liver Int. 2015;35:232-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 44. | Sanai FM, Sobki S, Bzeizi KI, Shaikh SA, Alswat K, Al-Hamoudi W, Almadi M, Al Saif F, Abdo AA. Assessment of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma in Middle Eastern patients. Dig Dis Sci. 2010;55:3568-3575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432-438. [PubMed] [Cited in This Article: ] |
| 46. | Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 2001;313:15-19. [PubMed] [Cited in This Article: ] |
| 47. | Leerapun A, Suravarapu SV, Bida JP, Clark RJ, Sanders EL, Mettler TA, Stadheim LM, Aderca I, Moser CD, Nagorney DM. The utility of Lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a United States referral population. Clin Gastroenterol Hepatol. 2007;5:394-402; quiz 267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Kusaba T. Relationship between Lens culinaris agglutinin reactive alpha-fetoprotein and biological features of hepatocellular carcinoma. Kurume Med J. 1998;45:113-120. [PubMed] [Cited in This Article: ] |
| 49. | Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F, Satomura S. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein & lt; 20 ng/mL. Cancer Sci. 2011;102:1025-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Yamamoto K, Imamura H, Matsuyama Y, Kume Y, Ikeda H, Norman GL, Shums Z, Aoki T, Hasegawa K, Beck Y. AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J Gastroenterol. 2010;45:1272-1282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Tanwandee T, Setthasin S, Charatcharoenwitthaya P, Chainuvati S, Leelakusolvong S, Pausawasdi N, Srikureja W, Pongprasobchai S, Manatsathit S, Kachintorn U. Clinical utility of lens culinaris agglutinin-reactive alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: evaluation in a Thai referral population. J Med Assoc Thai. 2009;92 Suppl 2:S49-S56. [PubMed] [Cited in This Article: ] |
| 52. | Hu B, Tian X, Sun J, Meng X. Evaluation of individual and combined applications of serum biomarkers for diagnosis of hepatocellular carcinoma: a meta-analysis. Int J Mol Sci. 2013;14:23559-23580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 53. | Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 462] [Cited by in F6Publishing: 502] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 54. | Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, Osaki Y, Seki T, Kudo M, Tanaka M. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378-1383. [PubMed] [Cited in This Article: ] |
| 55. | Xu WJ, Guo BL, Han YG, Shi L, Ma WS. Diagnostic value of alpha-fetoprotein-L3 and Golgi protein 73 in hepatocellular carcinomas with low AFP levels. Tumour Biol. 2014;35:12069-12074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Lefrere JJ, Gozin D. Use of des-gamma-carboxyprothrombin in retrospective diagnosis of hidden intoxication of anticoagulants. J Clin Pathol. 1987;40:589. [PubMed] [Cited in This Article: ] |
| 57. | Fujiyama S, Morishita T, Hashiguchi O, Sato T. Plasma abnormal prothrombin (des-gamma-carboxy prothrombin) as a marker of hepatocellular carcinoma. Cancer. 1988;61:1621-1628. [PubMed] [Cited in This Article: ] |
| 58. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 383] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Yu R, Ding S, Tan W, Tan S, Tan Z, Xiang S, Zhou Y, Mao Q, Deng G. Performance of Protein Induced by Vitamin K Absence or Antagonist-II (PIVKA-II) for Hepatocellular Carcinoma Screening in Chinese Population. Hepat Mon. 2015;15:e28806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Huang TS, Shyu YC, Turner R, Chen HY, Chen PJ. Diagnostic performance of alpha-fetoprotein, lens culinaris agglutinin-reactive alpha-fetoprotein, des-gamma carboxyprothrombin, and glypican-3 for the detection of hepatocellular carcinoma: a systematic review and meta-analysis protocol. Syst Rev. 2013;2:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 62. | Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, Gerken G, Schlaak JF. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Zhu R, Yang J, Xu L, Dai W, Wang F, Shen M, Zhang Y, Zhang H, Chen K, Cheng P. Diagnostic Performance of Des-γ-carboxy Prothrombin for Hepatocellular Carcinoma: A Meta-Analysis. Gastroenterol Res Pract. 2014;2014:529314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279-303. [PubMed] [Cited in This Article: ] |
| 65. | Wan HG, Xu H, Gu YM, Wang H, Xu W, Zu MH. Comparison osteopontin vs AFP for the diagnosis of HCC: a meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:706-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 66. | Khalil A, Elgedawy J, Faramawi MF, Elfert A, Salama I, Abbass A, Elsaid H, Elsebaai H. Plasma osteopontin level as a diagnostic marker of hepatocellular carcinoma in patients with radiological evidence of focal hepatic lesions. Tumori. 2013;99:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 67. | Ba MC, Long H, Tang YQ, Cui SZ. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol. 2012;5:874-881. [PubMed] [Cited in This Article: ] |
| 68. | Kladney RD, Bulla GA, Guo L, Mason AL, Tollefson AE, Simon DJ, Koutoubi Z, Fimmel CJ. GP73, a novel Golgi-localized protein upregulated by viral infection. Gene. 2000;249:53-65. [PubMed] [Cited in This Article: ] |
| 69. | Kladney RD, Cui X, Bulla GA, Brunt EM, Fimmel CJ. Expression of GP73, a resident Golgi membrane protein, in viral and nonviral liver disease. Hepatology. 2002;35:1431-1440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 176] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 70. | Marrero JA, Romano PR, Nikolaeva O, Steel L, Mehta A, Fimmel CJ, Comunale MA, D’Amelio A, Lok AS, Block TM. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol. 2005;43:1007-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 71. | Xu Z, Liu L, Pan X, Wei K, Wei M, Liu L, Yang H, Liu Q. Serum Golgi protein 73 (GP73) is a diagnostic and prognostic marker of chronic HBV liver disease. Medicine (Baltimore). 2015;94:e659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 3.8] [Reference Citation Analysis (3)] |
| 72. | Dai M, Chen X, Liu X, Peng Z, Meng J, Dai S. Diagnostic Value of the Combination of Golgi Protein 73 and Alpha-Fetoprotein in Hepatocellular Carcinoma: A Meta-Analysis. PLoS One. 2015;10:e0140067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Witjes CD, van Aalten SM, Steyerberg EW, Borsboom GJ, de Man RA, Verhoef C, Ijzermans JN. Recently introduced biomarkers for screening of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatol Int. 2013;7:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Jakubovic BD, Jothy S. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol. 2007;82:184-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [PubMed] [Cited in This Article: ] |
| 76. | Peters MG, Farías E, Colombo L, Filmus J, Puricelli L, Bal de Kier Joffé E. Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res Treat. 2003;80:221-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245-6254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 78. | Liu JW, Zuo XL, Wang S. Diagnosis accuracy of serum Glypican-3 level in patients with hepatocellular carcinoma and liver cirrhosis: a meta-analysis. Eur Rev Med Pharmacol Sci. 2015;19:3655-3673. [PubMed] [Cited in This Article: ] |
| 79. | Pontisso P, Calabrese F, Benvegnù L, Lise M, Belluco C, Ruvoletto MG, Marino M, Valente M, Nitti D, Gatta A. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer. 2004;90:833-837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 80. | Suminami Y, Nagashima S, Murakami A, Nawata S, Gondo T, Hirakawa H, Numa F, Silverman GA, Kato H. Suppression of a squamous cell carcinoma (SCC)-related serpin, SCC antigen, inhibits tumor growth with increased intratumor infiltration of natural killer cells. Cancer Res. 2001;61:1776-1780. [PubMed] [Cited in This Article: ] |
| 81. | Zhang J, Shao C, Zhou Q, Zhu Y, Zhu J, Tu C. Diagnostic accuracy of serum squamous cell carcinoma antigen and squamous cell carcinoma antigen-immunoglobulin M for hepatocellular carcinoma: A meta-analysis. Mol Clin Oncol. 2015;3:1165-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Fatima S, Lee NP, Luk JM. Dickkopfs and Wnt/β-catenin signalling in liver cancer. World J Clin Oncol. 2011;2:311-325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Rachner TD, Thiele S, Göbel A, Browne A, Fuessel S, Erdmann K, Wirth MP, Fröhner M, Todenhöfer T, Muders MH. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer. 2014;14:649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Gavriatopoulou M, Dimopoulos MA, Christoulas D, Migkou M, Iakovaki M, Gkotzamanidou M, Terpos E. Dickkopf-1: a suitable target for the management of myeloma bone disease. Expert Opin Ther Targets. 2009;13:839-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene. 2000;19:1843-1848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 86. | Zhang J, Zhao Y, Yang Q. Sensitivity and specificity of Dickkopf-1 protein in serum for diagnosing hepatocellular carcinoma: a meta-analysis. Int J Biol Markers. 2014;29:e403-e410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Kim SU, Park JH, Kim HS, Lee JM, Lee HG, Kim H, Choi SH, Baek S, Kim BK, Park JY. Serum Dickkopf-1 as a Biomarker for the Diagnosis of Hepatocellular Carcinoma. Yonsei Med J. 2015;56:1296-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 88. | Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 89. | Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14460] [Cited by in F6Publishing: 15327] [Article Influence: 1021.8] [Reference Citation Analysis (1)] |
| 90. | Hyun KA, Kim J, Gwak H, Jung HI. Isolation and enrichment of circulating biomarkers for cancer screening, detection, and diagnostics. Analyst. 2016;141:382-392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Huang JT, Liu SM, Ma H, Yang Y, Zhang X, Sun H, Zhang X, Xu J, Wang J. Systematic Review and Meta-Analysis: Circulating miRNAs for Diagnosis of Hepatocellular Carcinoma. J Cell Physiol. 2016;231:328-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 92. | Tu Y, Wan L, Fan Y, Wang K, Bu L, Huang T, Cheng Z, Shen B. Ischemic postconditioning-mediated miRNA-21 protects against cardiac ischemia/reperfusion injury via PTEN/Akt pathway. PLoS One. 2013;8:e75872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 93. | Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128-2136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1350] [Cited by in F6Publishing: 1417] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 94. | Huang CS, Yu W, Cui H, Wang YJ, Zhang L, Han F, Huang T. Increased expression of miR-21 predicts poor prognosis in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:7234-7238. [PubMed] [Cited in This Article: ] |
| 95. | Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu W, Yang K, He X, Chen S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatol. 2010;53:98-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 96. | Nakao K, Miyaaki H, Ichikawa T. Antitumor function of microRNA-122 against hepatocellular carcinoma. J Gastroenterol. 2014;49:589-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 97. | Wu K, Li L, Li S. Circulating microRNA-21 as a biomarker for the detection of various carcinomas: an updated meta-analysis based on 36 studies. Tumour Biol. 2015;36:1973-1981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 98. | Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799-2803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1136] [Cited by in F6Publishing: 1168] [Article Influence: 64.9] [Reference Citation Analysis (0)] |
| 99. | Arataki K, Hayes CN, Akamatsu S, Akiyama R, Abe H, Tsuge M, Miki D, Ochi H, Hiraga N, Imamura M. Circulating microRNA-22 correlates with microRNA-122 and represents viral replication and liver injury in patients with chronic hepatitis B. J Med Virol. 2013;85:789-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 100. | Li L, Gu X, Fang M, Ji J, Yi C, Gao C. The diagnostic value of serum fucosylated fetuin A in hepatitis B virus-related liver diseases. Clin Chem Lab Med. 2016;54:693-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Noh CK, Kim SS, Kim DK, Lee HY, Cho HJ, Yoon SY, Lee GH, Hyun SA, Kim YJ, Kim HJ. Inter-alpha-trypsin inhibitor heavy chain H4 as a diagnostic and prognostic indicator in patients with hepatitis B virus-associated hepatocellular carcinoma. Clin Biochem. 2014;47:1257-1261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Sun QK, Zhu JY, Wang W, Lv Y, Zhou HC, Yu JH, Xu GL, Ma JL, Zhong W, Jia WD. Diagnostic and prognostic significance of peroxiredoxin 1 expression in human hepatocellular carcinoma. Med Oncol. 2014;31:786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 103. | Qiao B, Wang J, Xie J, Niu Y, Ye S, Wan Q, Ye Q. Detection and identification of peroxiredoxin 3 as a biomarker in hepatocellular carcinoma by a proteomic approach. Int J Mol Med. 2012;29:832-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 104. | Nafee AM, Pasha HF, Abd El Aal SM, Mostafa NA. Clinical significance of serum clusterin as a biomarker for evaluating diagnosis and metastasis potential of viral-related hepatocellular carcinoma. Clin Biochem. 2012;45:1070-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Wang Y, Liu YH, Mai SJ, He LJ, Liao YJ, Deng HX, Guan XY, Zeng YX, Kung HF, Xie D. Evaluation of serum clusterin as a surveillance tool for human hepatocellular carcinoma with hepatitis B virus related cirrhosis. J Gastroenterol Hepatol. 2010;25:1123-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Zheng W, Yao M, Sai W, Qian Q, Pan L, Qiu L, Huang J, Wu W, Yao D. Diagnostic and prognostic significance of secretory clusterin expression in patients with hepatocellular carcinoma. Tumour Biol. 2016;37:999-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 107. | Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int. 2013;2013:136106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 218] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 108. | Reichl P, Fang M, Starlinger P, Staufer K, Nenutil R, Muller P, Greplova K, Valik D, Dooley S, Brostjan C. Multicenter analysis of soluble Axl reveals diagnostic value for very early stage hepatocellular carcinoma. Int J Cancer. 2015;137:385-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 109. | Fan JL, Yang YF, Yuan CH, Chen H, Wang FB. Circulating Tumor Cells for Predicting the Prognostic of Patients with Hepatocellular Carcinoma: A Meta Analysis. Cell Physiol Biochem. 2015;37:629-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 110. | Kelley RK, Magbanua MJ, Butler TM, Collisson EA, Hwang J, Sidiropoulos N, Evason K, McWhirter RM, Hameed B, Wayne EM. Circulating tumor cells in hepatocellular carcinoma: a pilot study of detection, enumeration, and next-generation sequencing in cases and controls. BMC Cancer. 2015;15:206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 111. | Yan J, Fan Z, Wu X, Xu M, Jiang J, Tan C, Wu W, Wei X, Zhou J. Circulating tumor cells are correlated with disease progression and treatment response in an orthotopic hepatocellular carcinoma model. Cytometry A. 2015;87:1020-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 113. | Wong VW, Janssen HL. Can we use HCC risk scores to individualize surveillance in chronic hepatitis B infection? J Hepatol. 2015;63:722-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 114. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 924] [Cited by in F6Publishing: 870] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 115. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2309] [Cited by in F6Publishing: 2210] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 116. | Chan HL, Tse CH, Mo F, Koh J, Wong VW, Wong GL, Lam Chan S, Yeo W, Sung JJ, Mok TS. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol. 2008;26:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 225] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 117. | Yang Y, Gao J, Li HL, Zheng W, Yang G, Zhang W, Ma X, Tan YT, Rothman N, Gao YT. Dose-response association between hepatitis B surface antigen levels and liver cancer risk in Chinese men and women. Int J Cancer. 2016;139:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Hsu CA, Kuo SF, Liu CH, Chen PJ. Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology. 2013;57:441-450. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 119. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140-1149.e3; quiz e13-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 371] [Cited by in F6Publishing: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 120. | Lao X, Ren S, Lu Y, Yang D, Qin X, Li S. Genetic polymorphisms of C-reactive protein increase susceptibility to HBV-related hepatocellular carcinoma in a Guangxi male population. Int J Clin Exp Pathol. 2015;8:16055-16063. [PubMed] [Cited in This Article: ] |
| 121. | Chanthra N, Payungporn S, Chuaypen N, Pinjaroen N, Poovorawan Y, Tangkijvanich P. Association of Single Nucleotide Polymorphism rs1053004 in Signal Transducer and Activator of Transcription 3 (STAT3) with Susceptibility to Hepatocellular Carcinoma in Thai Patients with Chronic Hepatitis B. Asian Pac J Cancer Prev. 2015;16:5069-5073. [PubMed] [Cited in This Article: ] |
| 122. | Chanthra N, Payungporn S, Chuaypen N, Piratanantatavorn K, Pinjaroen N, Poovorawan Y, Tangkijvanich P. Single Nucleotide Polymorphisms in STAT3 and STAT4 and Risk of Hepatocellular Carcinoma in Thai Patients with Chronic Hepatitis B. Asian Pac J Cancer Prev. 2015;16:8405-8410. [PubMed] [Cited in This Article: ] |
| 123. | Yao JY, Chao K, Li MR, Wu YQ, Zhong BH. Interleukin-21 gene polymorphisms and chronic hepatitis B infection in a Chinese population. World J Gastroenterol. 2015;21:4232-4239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Tan A, Gao Y, Yao Z, Su S, Jiang Y, Xie Y, Xian X, Mo Z. Genetic variants in IL12 influence both hepatitis B virus clearance and HBV-related hepatocellular carcinoma development in a Chinese male population. Tumour Biol. 2016;37:6343-6348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Zhang L, Xu K, Liu C, Chen J. Meta-analysis reveals an association of STAT4 polymorphism with hepatocellular carcinoma risk. Hepatol Res. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 126. | Jin F, Xiong WJ, Jing JC, Feng Z, Qu LS, Shen XZ. Evaluation of the association studies of single nucleotide polymorphisms and hepatocellular carcinoma: a systematic review. J Cancer Res Clin Oncol. 2011;137:1095-1104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 127. | Yao S, Johnson C, Hu Q, Yan L, Liu B, Ambrosone CB, Wang J, Liu S. Differences in somatic mutation landscape of hepatocellular carcinoma in Asian American and European American populations. Oncotarget. 2016; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 128. | Xu K, Meng XY, Wu JW, Shen B, Shi YC, Wei Q. Diagnostic value of serum gamma-glutamyl transferase isoenzyme for hepatocellular carcinoma: a 10-year study. Am J Gastroenterol. 1992;87:991-995. [PubMed] [Cited in This Article: ] |
| 129. | Lin YJ, Lee MH, Yang HI, Jen CL, You SL, Wang LY, Lu SN, Liu J, Chen CJ. Predictability of liver-related seromarkers for the risk of hepatocellular carcinoma in chronic hepatitis B patients. PLoS One. 2013;8:e61448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 130. | Tong MJ, Hsien C, Song JJ, Kao JH, Sun HE, Hsu L, Han SH, Durazo FA, Saab S, Blatt LM. Factors associated with progression to hepatocellular carcinoma and to death from liver complications in patients with HBsAg-positive cirrhosis. Dig Dis Sci. 2009;54:1337-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 131. | Oldberg A, Antonsson P, Lindblom K, Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267:22346-22350. [PubMed] [Cited in This Article: ] |
| 132. | Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31:583-591. [PubMed] [Cited in This Article: ] |
| 133. | Xiao Y, Kleeff J, Guo J, Gazdhar A, Liao Q, Di Cesare PE, Büchler MW, Friess H. Cartilage oligomeric matrix protein expression in hepatocellular carcinoma and the cirrhotic liver. J Gastroenterol Hepatol. 2004;19:296-302. [PubMed] [Cited in This Article: ] |
| 134. | Norman GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos GN. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol. 2015;7:1875-1883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 135. | Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766-2770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 136. | Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, Jung HS. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686-693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 137. | Ohishi W, Cologne JB, Fujiwara S, Suzuki G, Hayashi T, Niwa Y, Akahoshi M, Ueda K, Tsuge M, Chayama K. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014;134:154-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 138. | Cheng HY, Kang PJ, Chuang YH, Wang YH, Jan MC, Wu CF, Lin CL, Liu CJ, Liaw YF, Lin SM. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS One. 2014;9:e95870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 139. | Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300:281-290. [PubMed] [Cited in This Article: ] |
| 140. | Wang M, Block TM, Marrero J, Di Bisceglie AM, Devarajan K, Mehta A. Improved biomarker performance for the detection of hepatocellular carcinoma by inclusion of clinical parameters. Proceedings (IEEE Int Conf Bioinformatics Biomed). 2012;2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 141. | Shimauchi Y, Tanaka M, Kuromatsu R, Ogata R, Tateishi Y, Itano S, Ono N, Yutani S, Nagamatsu H, Matsugaki S. A simultaneous monitoring of Lens culinaris agglutinin A-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin as an early diagnosis of hepatocellular carcinoma in the follow-up of cirrhotic patients. Oncol Rep. 2000;7:249-256. [PubMed] [Cited in This Article: ] |
| 142. | Choi JY, Jung SW, Kim HY, Kim M, Kim Y, Kim DG, Oh EJ. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J Gastroenterol. 2013;19:339-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 69] [Cited by in F6Publishing: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 143. | Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054-1061. [PubMed] [Cited in This Article: ] |
| 144. | da Costa AN, Plymoth A, Santos-Silva D, Ortiz-Cuaran S, Camey S, Guilloreau P, Sangrajrang S, Khuhaprema T, Mendy M, Lesi OA. Osteopontin and latent-TGF β binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int J Cancer. 2015;136:172-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 145. | Yang JD, Gyedu A, Afihene MY, Duduyemi BM, Micah E, Kingham TP, Nyirenda M, Nkansah AA, Bandoh S, Duguru MJ. Hepatocellular Carcinoma Occurs at an Earlier Age in Africans, Particularly in Association With Chronic Hepatitis B. Am J Gastroenterol. 2015;110:1629-1631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 146. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 724] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 147. | He QY, Zhu R, Lei T, Ng MY, Luk JM, Sham P, Lau GK, Chiu JF. Toward the proteomic identification of biomarkers for the prediction of HBV related hepatocellular carcinoma. J Cell Biochem. 2008;103:740-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 148. | Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005;5:4581-4588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 149. | King LY, Canasto-Chibuque C, Johnson KB, Yip S, Chen X, Kojima K, Deshmukh M, Venkatesh A, Tan PS, Sun X. A genomic and clinical prognostic index for hepatitis C-related early-stage cirrhosis that predicts clinical deterioration. Gut. 2015;64:1296-1302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 150. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 978] [Cited by in F6Publishing: 962] [Article Influence: 60.1] [Reference Citation Analysis (0)] |