Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.954
Revised: June 10, 2004
Accepted: July 11, 2004
Published online: February 21, 2005
AIM: To investigate the possibility of recombinant high-density lipoprotein (rHDL) being a carrier for delivering antitumoral drug to hepatoma cells.
METHODS: Recombinant complex of HDL and aclacinomycin (rHDL-ACM) was prepared by cosonication of apoproteins from HDL (Apo HDL) and ACM as well as phosphatidylcholine. Characteristics of the rHDL-ACM were elucidated by electrophoretic mobility, including the size of particles, morphology and entrapment efficiency. Binding activity of rHDL-ACM to human hepatoma cells was determined by competition assay in the presence of excess native HDL. The cytotoxicity of rHDL-ACM was assessed by MTT method.
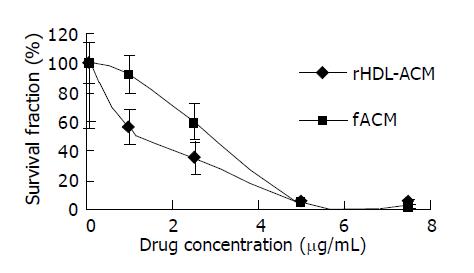
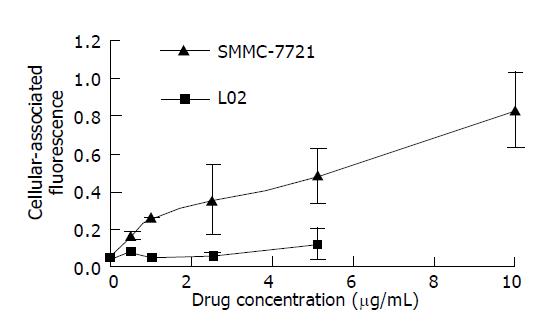
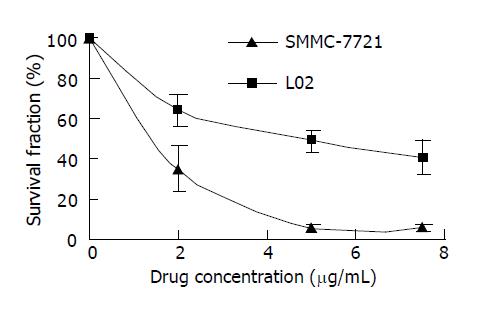
RESULTS: The density range of rHDL-ACM was 1.063-1.210 g/mL, and the same as that of native HDL. The purity of all rHDL-ACM preparations was more than 92%. Encapsulated efficiencies of rHDL-ACM were more than 90%. rHDL-ACM particles were typical sphere model of lipoproteins and heterogeneous in particle size. The average diameter was 31.26±5.62 nm by measure of 110 rHDL-ACM particles in the range of diameter of lipoproteins. rHDL-ACM could bind on SMMC-7721 cells, and such binding could be competed against in the presence of excess native HDL. rHDL-ACM had same binding capacity as native HDL. The cellular uptake of rHDL-ACM by SMMC-7721 hepatoma cells was significantly higher than that of free ACM at the concentration range of 0.5-10 µg/mL (P<0.01). Cytotoxicity of rHDL-ACM to SMMC-7721 cells was significantly higher than that of free ACM at concentration range of less than 5 µg/mL (P<0.01) and IC50 of rHDL-ACM was lower than IC50 of free ACM (1.68 nmol/L vs 3 nmol/L). Compared to L02 hepatocytes, a normal liver cell line, the cellular uptake of rHDL-ACM by SMMC-7721 cells was significantly higher (P<0.01) and in a dose-dependent manner at the concentration range of 0.5-10 μg/mL. Cytotoxicity of the rHDL-ACM to SMMC-7721 cells was significantly higher than that to L02 cells at concentration range of 1-7.5 μg/mL (P<0.01). IC50 for SMMC-7721 cells (1.68 nmol/L) was lower than that for L02 cells (5.68 nmol/L), showing a preferential cytotoxicity of rHDL-ACM for SMMC-7721 cells.
CONCLUSION: rHDL-ACM complex keeps the basic physical and biological binding properties of native HDL and shows a preferential cytotoxicity for SMMC-7721 hepatoma to normal L02 hepatocytes. HDL is a potential carrier for delivering lipophilic antitumoral drug to hepatoma cells.
- Citation: Lou B, Liao XL, Wu MP, Cheng PF, Yin CY, Fei Z. High-density lipoprotein as a potential carrier for delivery of a lipophilic antitumoral drug into hepatoma cells. World J Gastroenterol 2005; 11(7): 954-959
- URL: https://www.wjgnet.com/1007-9327/full/v11/i7/954.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.954
Clinical efficacy of anti-tumoral drug treatment is still a big challenge, despite several decades of intensive research. There are a number of factors, which we have to face, such as lack of specificity and development of drug resistance. Several strategies have been explored to overcome these problems. The use of liposomes, for example, has been studied as a delivery system to improve the efficacy of antitumoral drugs. However, liposomes have been found to be quickly cleared by reticuloendothelial system[1,2] and its targeting -efficiency is uncertain[3]. Another approach is using specific antibodies (Abs) to deliver the drugs. But most antibodies are murine in origin and, can be immunogenic, resulting in the production of antimouse protein Abs[4]. Lipoproteins are potential candidates for the delivery of drugs to cancer- cells[5]. Lipoproteins, heterogeneous in particle size, lipid and apolipoprotein contents, are classified as chylomicrons (CM), very low density lipoprotein (VLDL), low density lipoprotein (LDL), and high density lipoprotein (HDL). There are several advantages of lipoproteins as anti-tumoural drug carriers: (1) lipoproteins are spherical particles consisting of a core of apolar lipids surrounded by a phospholipid monolayer, in which cholesterol and apoproteins are embedded[6]. Highly lipophilic drugs can be incorporated into the apolar core[7,8] without affecting lipoprotein receptor recognition; (2) lipoproteins can be recognized and taken up via specific receptors, and can mediate cellular uptake of the carried drugs[1,2,9]; (3) being endogenous, lipoproteins are completely bio-degradable, do not trigger immunological responses, escape from recognition and elimination by the reticuloendothelial system (RES), and have a relatively long half-life in the circulation[10]; (4) many cancer -cells including the most aggressive ones show a high ability of lipoprotein uptake. This is presumably due to enhanced requirements for structural cholesterol and steroid-derived products in highly proliferative cells[11].
Many researches have been conducted to evaluate the lipoprotein-drug delivery system. It was reported that VLDL-drug complexes had the same cytotoxicity as free drugs. However, the authors did not evidence receptor-mediated uptake.[10,12]. A large number of investigations were concentrated on LDL-drug complex[1,2,8]. LDLs, which have great potential to store lipophilic molecules in their cores, have been extensively studied as drug- carriers and have been shown to be effective in improving efficacy and/or reducing toxicity of therapeutical agents[1,15]. Some carcinoma cell -lines have been reported to express more LDL receptors than normal cells[16,17]. Most of tumor- cells readily internalize and degrade LDL by the high-affinity receptor pathway and then drugs are released from LDL particles into the cells[12,18,19]. However, LDL receptors present in most normal tissue cells, may not solve the problem of specificity.
Recently, HDL has been explored as a drug -carrier system for a hydrophobic prodrug of IUdR[20] and for cervical and breast cancer chemotherapy[12]. HDL plays a major role in the transport of cholesterol from peripheral tissues to the liver (called ‘reverse cholesterol transport’)[21]. HDL transports cholesterol to liver cells, where they are recognized and taken up via specific receptors. Cholesteryl esters within HDL are selectively uptaken by hepatocytes via the scavenger receptor BI(SR-BI)[22,23]. An interesting feature of SR-BI is that the receptor selectively translocates HDL-cholesteryl esters from the lipoprotein particle to the cytosol of the liver parenchymal cells without a parallel uptake of the apolipoproteins and this property may allow for the delivery of its loaded drugs avoiding lysosomal degradation[24]. The ectopic β-chain of ATP synthase[25], as a new hepatic apoA-I receptor, and hepatic lipase[26] in the surface of hepatocytes may also take part in the selective uptake of HDL-cholesteryl esters. The rate of selective cellular uptake of cholesteryl ester by liver can be up to 40-fold higher than the uptake of apolipoprotein[27]. The lysosomal pathway of endocytic LDL may result in the destruction of its carried drugs[13,14]. As a drug carrier, non-lysosomal pathway of endocytic HDL[24,26] may be more desirable. As a drug delivery system, HDL may be better than other lipoproteins in hepatoma chemotherapy.
In this paper, we chose HDL as a drug carrier to explore the possibility of HDL-ACM complex in hepatoma chemotherapy. We found that rHDL-ACM complex keeps the basic physical and biological binding properties of native HDL and shows a preferential cytotoxicity for SMMC-7721 hepatoma to normal L02 hepatocytes.
Aclacinomycin (ACM) was purchased from Yangzhou Pharmaceutical Corporation (Jiangsu, China). Soy phosphatidylcholine was purchased from Shanghai Boao Co. Human hepatocellular carcinoma SMMC-7721 cells (SMMC-7721 cells) and hepatocyte L02, a normal liver cell line, was provided by Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science.
Isolation of high-density lipoprotein Human HDL (1.063-1.210 g/mL) was isolated by ultracentrifugation as previously described[28]. The isolated HDL was dialyzed at 4 °C against 0.15 mol/L NaCl, 1 mmol/L sodium EDTA, 0.02% NaN3, and pH 6.5.
Preparation of ApoHDL HDL was concentrated with polyethylene glycol 20000. The concentration of HDL protein was adjusted to 10 mg/mL. HDL was delipidated with ethanol: diethyl ether (3:2) mixture at -10 °C. Details of the procedure were described by Scanu[29]. The ApoHDL was collected, dried in N2 and stored at -20 °C.
Using HDL reconstitution technique[30] with some modification, we prepared rHDL-ACM complex. Soy phosphatidylcholine (80 mg), and ACM (3 mg) dispersed in 10 mL of 0.01 mol/L pH 8.0 Tris buffer (containing 0.1 mol/L KCl, 1 mmol/L EDTA and 0.02% NaN3) were sonicated using a probe sonicator for 30 min at room temperature. Then 10 mg apoA-I, dissolved in 2.5 mol/L urea, was added over a period of 5 min. Sonication was continued for another 10 min. The preparation was purified by density gradient centrifugation at 166200 g for 20 h and 1.063-1.210 g/mL density fractions were pooled. rHDL-ACM was exhaustively dialyzed against 0.15 mol/L NaCl, 1 mmol/L sodiumEDTA, 0.02% NaN3, and pH 6.5.
The prepared rHDL-ACM was purified on SephadexG-25 (1×18 cm) after dialyzed against 0.15 mol/L NaCl, 1 mmol/L EDTA, 0.02% NaN3, pH 6.5 to remove free ACM. rHDL-ACM was stored at 4 °C.
One hundred microliter Protamin sulfate (10 mg/mL) was added to 0.5 mL purified rHDL-ACM (phosphatidylcholine/Protamin sulfate mass ratio 2:1), mixed for 3 min at room temperature and spun by centrifugation at 1 920 g for 30 min. The precipitate was extracted by 1 mL chloroform: methanol (1:1) mixture. The ACM in organic phase was determined by UV at wavelength of 431 nm. The content of ACM encapsulated in rHDL-ACM can be calculated according to standard curve of ACM. Another part of purified 0.5 mL rHDL-ACM was added into 2 mL chloroform/methanol (1:1) mixture and shaken for 2 min. The content of ACM in organic phase (total content of ACM in rHDL-ACM solution) was measured and calculated according to standard curve of ACM.
Purity of rHDL-ACM(%) = the content of ACM encapsulated in rHDL-ACM/the total content of ACM in the purified rHDL-ACM solution ×100%.
Encapsulated efficiency(%) = (the content of ACM encapsulated in rHDL-ACM/the total amount of added ACM) ×100%.
Electrophoretic study The electrophoretic mobility of the rHDL-ACM complex and native HDL were compared on agarose gel electrophoresis[31].
The size and morphology of rHDL-ACM The mean diameter of the rHDL-ACM particles was obtained by Zata Potential/Particle Sizer (Nicomp 380 ZlS). rHDL-ACM particles were stained with 2% potassium phosphotungstate pH 7.4 and then the morphology was observed under transmission electron microscope (Philip Co.). The magnification was 52 K.
Competitive binding assay of rHDL-ACM to SMMA-7721 cells SMMC-7721 cells were routinely cultured in RPMI 1640 (gibco) containing penicillin (100 IU/mL), streptomycin (100 μg/mL) and 100 mL/L calf serum and maintained in a humidified atmosphere containing 50 mL/L CO2 at 37 °C. Cells were seeded in 6-well plates at a concentration of 4×105 cells/well. After incubation for 24 h, the medium was removed and replaced with the media supplemented with rHDL-ACM at a final concentration of 125 μg/mL and incubated with an excess of native HDL with a final concentration of 0, 125, 250, 500, 1000, 2000 μg/mL. The assays were performed in triplicate. After incubation for 3 h at 37 °C, the medium was removed and cells were washed with pH 7.2 PBS for three times and then dissolved in 1 mL 0.4% NaOH, 2 mL of chloroform: methanol (1:1) mixture was added to each well. The plate was then agitated on an orbital shaker for 2 min and centrifuged at 1920 g for 10 min. The fluorescent absorbance of ACM in organic phase was measured using an excitation wave at 440 nm and the emission wave at 560 nm. All assays were performed in triplicate.
SMMC-7721 cells were cultured and seeded. The procedures were as described above. The concentration of SMMC-7721 cells was 4×105 cells/well. After 24 h culture, the medium was replaced with the media supplemented with rHDL-ACM or free ACM with concentrations of 0, 0.5, 1.0, 2.5, 5.0, and 10 μg/mL. After incubating for 24 h, the medium was removed and cells were washed with pH 7.2 PBS for three times. ACM in cells was measured. All assays were performed in triplicate.
The procedures were described as above. The concentration of SMMC-7721 cells or hepatocyte L02 cells was 4×105 cells/well. The concentrations of rHDL-ACM were 0, 0.5, 1.0, 2.5, 5.0, and 10 μg/mL. After incubation for 24-h, ACM in SMMC-7721 cells or hepatocyte L02 cells was measured. All assays were performed in triplicate.
SMMC-7721 cells were cultured in 96-well- plates at a concentration of 1 000 cells/well. After 48 h of incubation, drugs (rHDL-ACM, free ACM) were added respectively at concentrations of 0.5, 1.0, 2.5, 5.0, and 7.5 μg/mL. The plates were incubated for 24 h at 37 °C and the media was removed. Cell survival- rate was measured by MTT assay[32]. All assays were performed in triplicate. The formula used to calculate the cell survival rate (%) was as follows: cell survival rate(%) = (net absorbance for cells treated with free ACM or rHDL-ACM/net absorbance for untreated cells) ×100%. IC50 of rHDL-ACM complex and free ACM to SMMC-7721 cells were calculated.
The procedures were the same as above. rHDL-ACM was added at the concentrations of 0, 0.5, 2.0, 5.0, and 7.5 μg/mL. All assays were performed in triplicate.
Statistical analysis was performed using Student’s t-test.
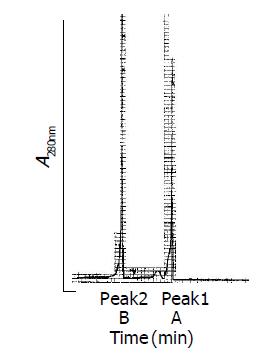
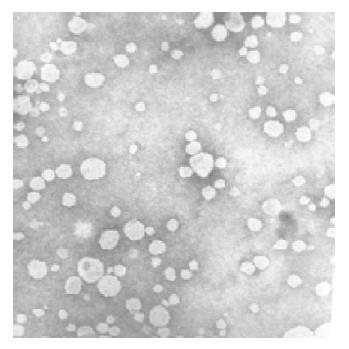
The rHDL-ACM was prepared by sonication, density gradient ultra-centrifugation and size fractionation. The density range of rHDL-ACM was 1.063-1.210 g/mL, the same as that of native HDL[33]. As shown in Figures 1A and 1B, there was no free ACM after dialysis. The purity of all rHDL-ACM preparations was more than 92%. Its encapsulated efficiencies were more than 90%. We further determined electrophoretic mobility of the complex and found that the mobility of rHDL-ACM on agarose gels was faster than that of native HDL (0.32 vs 0.16). Moreover, we examined the complex by using negative staining transmission electron microscopy and found that rHDL-ACM particles were typical sphere model of lipoproteins and heterogeneous in particle size. The average diameter was 31.26±5.62 nm by measure of 110 rHDL-ACM particles. This is consistent with the standard deviation for the average diameter of the particles that was determined by particle sizer (30.7±3.9 nm). rHDL-ACM particles were in the range of diameter of lipoproteins[33] (Figure 2).
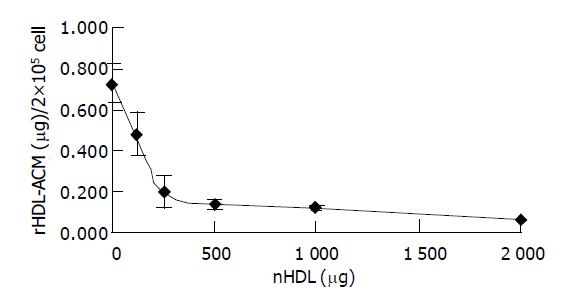
In order to determine whether rHDL-ACM still had same cell-binding activity as native HDL, we carried out a competitive binding assay. As shown in Figure 3, rHDL-ACM could bind to SMMC-7721 cells and such binding could be competed against in the presence of excess native HDL, indicating that rHDL-ACM complex still had native HDL property.
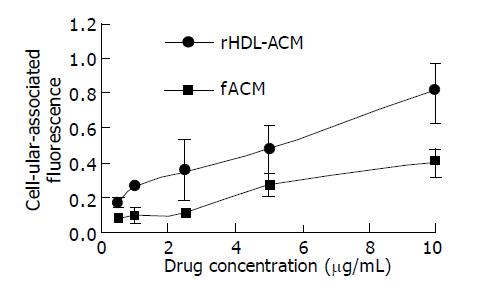
To test the drug delivery efficiency, we incubated the SMMC-7721 cells with increasing concentrations of rHDL-ACM or free ACM. As shown in Figure 4, rHDL-ACM complex delivered significantly higher amount of ACM into cells than free ACM at a concentration range of 0.5-10 µg/mL (P<0.01) revealing that rHDL-ACM complex had a high drug delivery efficiency.
We further evaluated the cytotoxicity and 50% inhibitory concentration (IC50) of rHDL-ACM and free ACM for SMMC-7721 cells. It was found that cytotoxicity of rHDL-ACM was significantly higher than that of free ACM at concentrations of less than 5 µg/mL (P<0.01) (Figure 5) and IC50 of rHDL-ACM was lower than that of free ACM (1.68 nmol/l vs 3 nmol/L).
In order to investigate the specificity of rHDL-ACM delivery system, we utilized both SMMC-7721 cells and L02 cells, and found that uptake of rHDL-ACM by SMMC-7721 cells was significantly higher than by L02 cells at a concentration range of 0.5-10 µg/mL (P<0.01) with a dose–dependent manner (Figure 6), suggesting that rHDL-ACM has some specificity to target the cancer cells. Finally, we determined cytotoxicity of rHDL-ACM for both SMMC-7721 and L02 cells. As shown in Figure 7, cytotoxicity of rHDL-ACM to SMMC-7721 cells was significantly higher than that ofL02 cells at a concentration range of 1-7.5 µg/mL (P<0.01). IC50 for SMMC-7721 cells (1.68 nmol/L) was lower than that ofL02 cells (5.68 nmol/L), showing a preferential cytotoxicity of rHDL-ACM for SMMC-7721 cells.
Among lipoproteins, only LDL has been explored intensively as a drug carrier for cancer chemotherapy[1,2,8]. In the present paper, we demonstrated that it was possible to use HDL as a carrier for a hydrophobic antitumoral drug (ACM) delivery. We found that (1) rHDL-ACM complexes were regular spherical particles and its encapsulated efficiencies were more than 90%; (2) rHDL-ACM had same binding capacity as native HDL; (3) the cellular uptake of rHDL-ACM by SMMC-7721 hepatoma cells was significantly higher than that of free ACM; (4) compared to L02 hepatocytes, a normal liver cell line, the cellular uptake of rHDL-ACM by SMMC-7721 cells was significantly higher; (5) rHDL-ACM had a higher cytotoxicity than that of free ACM; and (6) SMMC-7721 hepatoma cells were more sensitive to rHDL-ACM than L02 cells.
The lipid core of lipoprotein is the better site, because it has a relatively larger capacity. Lipoproteins can sequester drugs from enzymes in the circulation and can incorporate drugs without affecting lipoprotein-receptor binding. In order to prepare rHDL-ACM complex, we added hydrophobic antitumoral drug (ACM) to reconstituted HDL (consisting of delipidated HDL and phosphatidylcholine) to substitute core lipid of HDL. Drugs were incorporated into lipoproteins without disrupting their integrity, which is important for their recognition by receptors[2,10,12]. It has been reported recently that SR-BI[22-24], ectopic β-chain of ATP synthase[25], as well as hepatic lipase[26] take part in the endocytosis of HDL and the selective uptake of HDL-cholesteryl ester. Our results showed that rHDL-ACM complex keeps the typical spherical particles of plasma lipoproteins and its average diameter (31.3±5.6 nm) is in the range of diameter of plasma lipoprotein[33]. The density range of rHDL-ACM (1.063-1.210 g/mL) is the same as native HDL. Although a higher molar ratio of phosphatidylcholine/ApoA-I in rHDL-ACM might result in its faster mobility than in native HDL, it still keeps cell- binding activity of native plasma HDL. This is important for the potential use of HDL as a drug carrier, since success would depend on the preservation of its receptor recognition. These results showed that rHDL-ACM does not change the integrity of native HDL.
We studied the possibility of reconstituted HDL particles as drug- carriers in vitro for the special delivery of ACM to hepatoma cell SMMC-7721. The human normal hepatocyte L02 cells were used as control cells. The cellular uptake of ACM encapsulated in rHDL was higher than free ACM by SMMC-7721 cells (P<0.01). The uptake of rHDL-ACM by SMMC-7721 cells was higher as compared to L02 cells (P<0.01). Furthermore, uptake of rHDL-ACM by SMMC-7721 cells was dose-dependent. Compared to free ACM, cytotoxicity of rHDL-ACM against SMMC-7721 cells increased 1.79-fold. When compared with control L02 cells, the cytotoxicity of rHDL-ACM to SMMC-7721 cells increased 3.38-fold. These results suggest that rHDL-ACM complex could specifically deliver anti-tumor drug ACM to hepatoma cells SMMC-7721.
Cancer cells, which proliferate rapidly, need large amounts of cholesterol for synthesis of new cell membranes. It was reported that some carcinoma cell- lines express more LDL receptors than normal cells[16,17]. Our results showed hepatoma- cells had an increased uptake of rHDL-ACM as compared to L02 cells. All these imply that hepatoma cells could have more HDL receptors than normal cell types.
ACM, a lipophilic anti-tumor anthracycline antibiotic was developed for the purpose of reducing the cardiotoxicity of anthracyclines. Our results showed that HDL, as a liver targeting carrier, could combine with ACM to form a rHDL-ACM complex. The complex possesses higher selectivity to hepatoma cells and potent application for targeting therapy of hepatoma. The pharmacokinetics studies of HDL-ACM in vivo are in progress.
| 1. | Firestone RA. Low-density lipoprotein as a vehicle for targeting antitumor compounds to cancer cells. Bioconjug Chem. 1994;5:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 183] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | de Smidt PC, van Berkel TJ. LDL-mediated drug targeting. Crit Rev Ther Drug Carrier Syst. 1990;7:99-120. [PubMed] [Cited in This Article: ] |
| 3. | Janknegt R. Liposomal formulations of cytotoxic drugs. Support Care Cancer. 1996;4:298-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Khazaeli MB, Conry RM, LoBuglio AF. Human immune response to monoclonal antibodies. J Immunother Emphasis Tumor Immunol. 1994;15:42-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 213] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Tokui T, Kuroiwa C, Muramatsu S, Tokui Y, Sasagawa K, Ikeda T, Komai T. Plasma lipoproteins as targeting carriers to tumour tissues after administration of a lipophilic agent to mice. Biopharm Drug Dispos. 1995;16:91-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Hauser H, Kostner GM. Structural organization of free and esterified cholesterol in human high density lipoproteins. A 100.6 MHz 13C NMR study. Biochim Biophys Acta. 1979;573:375-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Samadi-Baboli M, Favre G, Canal P, Soula G. Low density lipoprotein for cytotoxic drug targeting: improved activity of elliptinium derivative against B16 melanoma in mice. Br J Cancer. 1993;68:319-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Kader A, Davis PJ, Kara M, Liu H. Drug targeting using low density lipoprotein (LDL): physicochemical factors affecting drug loading into LDL particles. J Control Release. 1998;55:231-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Filipowska D, Filipowski T, Morelowska B, Kazanowska W, Laudanski T, Lapinjoki S, Akerlund M, Breeze A. Treatment of cancer patients with a low-density-lipoprotein delivery vehicle containing a cytotoxic drug. Cancer Chemother Pharmacol. 1992;29:396-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Pussinen PJ, Lindner H, Glatter O, Reicher H, Kostner GM, Wintersperger A, Malle E, Sattler W. Lipoprotein-associated alpha-tocopheryl-succinate inhibits cell growth and induces apoptosis in human MCF-7 and HBL-100 breast cancer cells. Biochim Biophys Acta. 2000;1485:129-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Lenz M, Miehe WP, Vahrenwald F, Bruchelt G, Schweizer P, Girgert R. Cholesterol based antineoplastic strategies. Anticancer Res. 1997;17:1143-1146. [PubMed] [Cited in This Article: ] |
| 12. | Kader A, Pater A. Loading anticancer drugs into HDL as well as LDL has little affect on properties of complexes and enhances cytotoxicity to human carcinoma cells. J Control Release. 2002;80:29-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Masquelier M, Tirzitis G, Peterson CO, Pålsson M, Amolins A, Plotniece M, Plotniece A, Makarova N, Vitols SG. Plasma stability and cytotoxicity of lipophilic daunorubicin derivatives incorporated into low density lipoproteins. Eur J Med Chem. 2000;35:429-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Masquelier M, Vitols S, Pålsson M, Mårs U, Larsson BS, Peterson CO. Low density lipoprotein as a carrier of cytostatics in cancer chemotherapy: study of stability of drug-carrier complexes in blood. J Drug Target. 2000;8:155-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Masquelier. Method for the production of a macromolecular carrier loaded with a biologically active substance. United States Patent 1989; September 19. . [Cited in This Article: ] |
| 16. | Lundberg B. Assembly of prednimustine low-density-lipoprotein complexes and their cytotoxic activity in tissue culture. Cancer Chemother Pharmacol. 1992;29:241-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Xiao W, Wang L, Ryan JM, Pater A, Liu H. Incorporation of an (125)I-labeled hexa-iodinated diglyceride analog into low-density lipoprotein and high specific uptake by cells of cervical carcinoma cell lines. Radiat Res. 1999;152:250-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897-930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1893] [Cited by in F6Publishing: 1836] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 19. | Lundberg B, Suominen L. Preparation of biologically active analogs of serum low density lipoprotein. J Lipid Res. 1984;25:550-558. [PubMed] [Cited in This Article: ] |
| 20. | Bijsterbosch MK, Schouten D, van Berkel TJ. Synthesis of the dioleoyl derivative of iododeoxyuridine and its incorporation into reconstituted high density lipoprotein particles. Biochemistry. 1994;33:14073-14080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Pieters MN, Schouten D, Van Berkel TJ. In vitro and in vivo evidence for the role of HDL in reverse cholesterol transport. Biochim Biophys Acta. 1994;1225:125-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1766] [Cited by in F6Publishing: 1735] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 23. | Acton SL, Kozarsky KF, Rigotti A. The HDL receptor SR-BI: a new therapeutic target for atherosclerosis? Mol Med Today. 1999;5:518-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Rinninger F, Brundert M, Budzinski RM, Fruchart JC, Greten H, Castro GR. Scavenger receptor BI (SR-BI) mediates a higher selective cholesteryl ester uptake from LpA-I compared with LpA-I: A-II lipoprotein particles. Atherosclerosis. 2003;166:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Tercé F. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 26. | Wei Q, Wu MP, Chen PF, Mei MZ, Gao XJ. Cooperation of HDL receptor and hepatic lipase in the selective uptake of HDL2-CE by rat hepatic sinusoidal cells. Shengwu Huaxue Yu Wuli Xuebao. 1996;28:659 663. [Cited in This Article: ] |
| 27. | Shaw JM, Shaw KV, Yanovich S, Iwanik M, Futch WS, Rosowsky A, Schook LB. Delivery of lipophilic drugs using lipoproteins. Ann N Y Acad Sci. 1987;507:252-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Schumaker VN, Puppione DL. Sequential flotation ultracentrifugation. Methods Enzymol. 1986;128:155-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 406] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 29. | Scanu A. Forms of human serum high density lipoprotein protein. J Lipid Res. 1966;7:295-306. [PubMed] [Cited in This Article: ] |
| 30. | Pittman RC, Glass CK, Atkinson D, Small DM. Synthetic high density lipoprotein particles. Application to studies of the apoprotein specificity for selective uptake of cholesterol esters. J Biol Chem. 1987;262:2435-2442. [PubMed] [Cited in This Article: ] |
| 31. | Eklund A, Sjöblom L. Improved banding pattern of rat plasma lipoproteins developed by agarose gel electrophoresis at pH 7.0. Biochim Biophys Acta. 1986;877:135-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Jabbar SA, Twentyman PR, Watson JV. The MTT assay underestimates the growth inhibitory effects of interferons. Br J Cancer. 1989;60:523-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Rensen PC, de Vrueh RL, Kuiper J, Bijsterbosch MK, Biessen EA, van Berkel TJ. Recombinant lipoproteins: lipoprotein-like lipid particles for drug targeting. Adv Drug Deliv Rev. 2001;47:251-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |