Cylindrical Layered Bone Scaffolds with Anisotropic Mechanical Properties as Potential Drug Delivery Systems
Abstract
:1. Introduction
2. Results
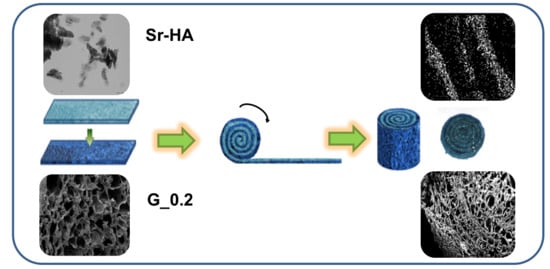
2.1. Sr-HA Characterization
2.2. Scaffold Characterization
2.3. Mechanical Properties
2.4. Extent of Crosslinking
2.5. Water Uptake Ability (WUA)
2.6. Gelatin Release
2.7. Strontium Release
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Scaffolds Preparation
4.3. X-ray Powder Diffraction
4.4. Morphological Characterizations
4.5. Mechanical Properties
4.6. Extent of Crosslinking
4.7. Water Uptake Ability (WUA)
4.8. Gelatin Release
4.9. Strontium Release
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; De Pollak, C.; Bourguignon, M. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Bongio, M.; van den Beucken, J.J.; Leeuwenburgh, S.C.; Jansen, J.A. Development of bone substitute materials: From ‘biocompatible’ to ‘instructive’. J. Mater. Chem. 2010, 20, 8747–8759. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomaterialia 2018, 80, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Gonzalez, B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, X.; Wang, Y.; Ortiz, L.; Mao, X.; Jiang, Z.; Xiao, Z.; Huang, N. Nanofiber scaffold with gradients in mineral content for spatial control of osteogenesis. ACS Appl. Mater. Interfaces 2013, 5, 319–330. [Google Scholar] [CrossRef]
- Babaie, E.; Bhaduri, S.B. Fabrication Aspects of Porous Biomaterials in Orthopedic Applications: A Review. ACS Biomater. Sci. Eng. 2018, 4, 1–39. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ferrer, M.L.; Gutiérrez, M.C.; Del Monte, F.; Ruiz-Hitzky, E. Progress in bionanocomposite and bioinspired foams. Adv. Mater. 2011, 23, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Soundarya, S.P.; Haritha Menon, A.; Viji Chandran, S.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.B.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interfaces 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomaterialia 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. Biomater. Appl. Nanomed. IntechOpen 2011. [Google Scholar] [Green Version]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA; London, UK, 1964. [Google Scholar]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple helix content and mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef]
- Gòmez-Guillén, M.C.; Giménez, B.; Lòpez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813. [Google Scholar] [CrossRef]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670. [Google Scholar] [CrossRef]
- Rault, J.; Herbage, F.D.; Abdul-Malak, N.; Huc, A. Evaluation of different chemical methods for crosslinking collagen gels, films and sponges. J. Mater. Sci. Mater. Med. 1996, 7, 215–221. [Google Scholar] [CrossRef]
- Sung, H.W.; Liang, I.L.; Chen, C.N.; Huang, R.N.; Liang, H.F. Stability of a biological tissue fixed with a naturally occurring cross-linking agent (genipin). J. Biomed. Mater. Res. 2011, 55, 538–546. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Yao, C.H.; Liu, B.S.; Chang, C.J.; Hsu, S.H.; Chen, Y.S. Preparation of networks of gelatin and genipin as degradable biomaterials. Mater. Chem. Phys. 2004, 83, 204–208. [Google Scholar] [CrossRef]
- Huang, K.S.; Lu, K.; Yeh, C.S.; Chung, S.R.; Lin, C.H.; Yang, C.H.; Dong, Y.S. Microfluidic controlling monodisperse microdroplet for 5-fluorouracil loaded genipin-gelatin microcapsules. J. Control. Release 2009, 137, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D.; Dhillon, S. Strontium ranelate: A review on its use in the treatment of postmenopausal osteoporosis. Drugs 2010, 70, 733. [Google Scholar] [CrossRef] [PubMed]
- Gallacher, S.J.; Dixon, T. Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate and denosumab) on bone quality: A systematic review. Calcif. Tissue Int. 2010, 87, 469. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: A novel mode of action optimizing bone formation and resorption. Osteoporosis Int. 2005, 16, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Ammann, P. Strontium ranelate: A novel mode of action leading to renewed bone quality. Osteoporosis Int. 2005, 16, S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Bruyère, O.; Sawicki, A.; Roces-Varela, A.; Fardellone, P.; Roberts, A.; Devogelaer, J.P. Long-term treatment of postmenopausal osteoporosis with strontium ranelate: Results at 8 years. Bone 2009, 45, 1059. [Google Scholar] [CrossRef]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216. [Google Scholar] [CrossRef]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium- substituted hydroxyapatite- gelatin biomimetic scaffolds modulate bone cell response. Macromol. Biosci. 2018. [Google Scholar] [CrossRef]
- Panzavolta, S.; Torricelli, P.; Sturba, L.; Bracci, B.; Giardino, R.; Bigi, A. Setting properties and in vitro bioactivity of strontium-enriched gelatin–calcium phosphate bone cements. J. Biomed. Mater. Res. A 2007. [Google Scholar] [CrossRef]
- Bracci, B.; Torricelli, P.; Panzavolta, S.; Boanini, E.; Giardino, R.; Bigi, A. Effect of Mg2+, Sr2+ and Mn2+ on the chemico-physical and in vitro biological properties of calcium phosphate biomimetic coatings. J. Inorg. Biochem. 2009, 103, 1666–1674. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis: A review. Acta Biomater. 2014, 10, 557. [Google Scholar] [CrossRef]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Tadier, S.; Bareille, R.; Siadous, R.; Marsan, O.; Charvillat, C.; Cazalbou, S.; Amédé, J.; Rey, C.; Combes, C. Strontium-loaded mineral bone cements as sustained release systems: Compositions, release properties, and effects on human osteoprogenitor cells. J. Biomed. Mater. Res. B 2012, 100, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Jiang, H.; Zou, Y.; Wang, H.; Du, J.; Li, Y.; Yang, X. Biomimetic spiral- cylindrical scaffold based on hybrid chitosan/cellulose/nano-hydroxyapatite membrane for bone regeneration. ACS App. Mater. Interfaces 2013, 5, 12036–12044. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Feng, G.; Shen, F.H.; Laurencin, C.T.; Li, X. Biphasic scaffold for annulus fibrosus tissue regeneration. Biomaterials 2008, 29, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Light, N.D.; Macgregor, J.; Harvey, W.W.; Paul, W. Absorbable Structures for Ligament and Tendon Repair. U.S. Patent 5,514,181, 7 May 1996. [Google Scholar]
- Berman, A.B. Resorbable Interposition Arthroplasty Implant. U.S. Patent 6,017,366, 25 January 2000. [Google Scholar]
- Sussman, M.B.; Zvi Kadouri, A. Fibrous Matrix for in vitro Cell Cultivation. U.S. Patent 5,266,476, 30 November 1993. [Google Scholar]
- Wang, J.; Shah, A.; Yu, X. The influence of fiber thickness, wall thickness and gap distance on the spiral nanofibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2011, 31, 50–56. [Google Scholar] [CrossRef]
- Dahl, S.G.; Allain, P.; Marie, P.J.; Mauras, Y.; Boivin, G.; Ammann, P.; Tsouderos, Y.; Delmas, P.D.; Christiansen, C. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef]
- Gioffrè, M.; Torricelli, P.; Panzavolta, S.; Rubini, K.; Bigi, A. Role of pH on stability and mechanical properties of gelatin films. J. Bioact. Compat. Pol. 2012, 27, 67. [Google Scholar] [CrossRef]
- Okuyama, K. Revisiting the molecular structure of collagen connective tissue. Tissue Res. 2008, 49, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Torricelli, P.; Panzavolta, S.; Parrilli, A.; Fini, M.; Bigi, A. Highly porous gelatin reinforced 3D scaffolds for articular cartilage regeneration. Macromol. Biosci. 2015. [Google Scholar] [CrossRef]
- Davidenko, N.; Gibb, T.; Schuster, C.; Best, S.M.; Campbell, J.J.; Watson, C.J.; Cameron, R.E. Biomimetic collagen scaffolds with anisotropic pore architecture. Acta Biomater. 2012, 8, 667–676. [Google Scholar] [CrossRef]
- Panzavolta, S.; Torricelli, P.; Amadori, S.; Parrilli, A.; Rubini, K.; della Bella, E.; Fini, M.; Bigi, A. 3D interconnected porous biomimetic scaffolds: In vitro cell response. J. Biomed. Mater. Res. A 2013, 101, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Panzavolta, S.; Fini, M.; Nicoletti, A.; Bracci, B.; Rubini, K.; Giardino, R.; Bigi, A. Porous composite scaffolds based on gelatin and partially hydrolyzed α-tricalcium phosphate. Acta Biomater. 2009, 5, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Bjurhager, I.; Malho, J.M.; Pere, J.; Ruokolainen, J.; Berglund, L.A.; Ikkala, O. Large-area, lightweight and thick biomimetic composites with superior material properties via fast, economic, and green pathways. Nano Lett. 2010, 10, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.A.; Ghelashvili, M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys. J. 1999, 76, 3243–3252. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Homminga, J.; McCreadie, B.R.; Ciarelli, T.E.; Weinans, H.; Goldstein, S.A.; Huiskes, R. Cancellous bone mechanical properties from normals and patients with hip fractures differ on the structure level, not on the bone hard tissue level. Bone 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Giesena, E.B.W.; Dingb, M.; Dalstrab, M.; van Eijdena, T.M.G.J. Mechanical properties of cancellous bone in the human mandibular condyle are anisotropic. J. Biomech. 2001, 34, 799–803. [Google Scholar] [CrossRef]
- Amadori, S.; Torricelli, P.; Panzavolta, S.; Parrilli, A.; Fini, M.; Bigi, A. Multi-layered scaffolds for osteochondral tissue engineering: In vitro response of co-cultured human mesenchymal stem cells. Macromol. Biosci. 2015, 15, 1535–1545. [Google Scholar] [CrossRef]
- Tanimoto, Y.; Hayakawa, T.; Sakae, T.; Nemoto, K. Characterization and bioactivity of tap-cast and sintered TCP Sheets. J. Biomed. Mater. Res. Part A 2005, 76, 571–579. [Google Scholar]
- Hataka, T.; Sato, H.; Watanabe, Y.; Matsumoto, M. Effect of formaldehyde on the physiochemical properties of soft gelatin capsule shells. Chem. Pharm. Bull. 1994, 42, 1138–1142. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| Single-Layer Scaffolds | % of Genipin (w of Genipin/w of Gelatin) | |
| S_0.1_Sr-HA | 0.1 | |
| G_0.05 | 0.05 | |
| G_0.1 | 0.1 | |
| G_0.15 | 0.15 | |
| G_0.2 | 0.2 | |
| G_0.1_Sr-HA | 0.1 | |
| (a) | ||
| Double-Layer Scaffolds | % of Genipin (w of Genipin/w of Gelatin) | |
| Inner Layer | Outer Layer | |
| G_0.05/G_0.2 | 0.05 | 0.2 |
| G_0.1/G_0.2 | 0.1 | 0.2 |
| G_0.15/G_0.2 | 0.15 | 0.2 |
| G_0.1_Sr-HA/G_0.2 | 0.1 | 0.2 |
| (b) | ||
| Sample | (%) |
|---|---|
| G_0.05 | 8 ± 1 |
| G_0.1 | 14 ± 3 |
| G_0.15 | 26 ± 2 |
| G_0.2 | 30 ± 1 |
| G_0.1_Sr-HA | 15 ± 3 |
| Sample | WUA (g PBS/g Gelatin) |
|---|---|
| S_0.1-Sr-HA | 1.7 ± 0.3 |
| G_0.05 | 3.1 ± 0.9 |
| G_0.1 | 2.2 ± 0.8 |
| G_0.15 | 2.2 ± 0.3 |
| G_0.2 | 2.1 ± 0.4 |
| G_0.1_Sr-HA | 1.8 ± 0.3 |
| G_0.1/G_0.2 | 5.0 ± 0.6 |
| G_0.15/G_0.2 | 6.9 ± 0.7 |
| G_0.1_Sr-HA/G_0.2 | 5.0 ± 0.4 |
| Sample | Time (Days) | ||||||
|---|---|---|---|---|---|---|---|
| 2 Days | 7 Days | 14 Days | 21 Days | 28 Days | 49 Days | 70 Days | |
| S_0.1_Sr-HA | 14.8 ± 0.1 | 22.7 ± 0.1 | 33.7 ± 2 | 39.3 ± 1.3 | 49 ± 2 | dissolved | dissolved |
| G_0.05 | 26 ± 2 | 47 ± 2 | dissolved | dissolved | dissolved | dissolved | dissolved |
| G_0.1 | 16 ± 1 | 26.9 ± 0.9 | 36.8 ± 0.4 | 44.3 ± 0.8 | 52.1 ± 0.6 | dissolved | dissolved |
| G_0.15 | 12 ± 1 | 20.7 ± 1 | 28.1 ± 0.4 | 34.6 ± 0.2 | 40.9 ± 0.2 | 66 ± 1 | dissolved |
| G_0.2 | 11 ± 1 | 17.6±0.8 | 23.7 ± 0.9 | 28.7 ± 0.5 | 32.7 ± 0.6 | 38.6 ± 0.6 | 56 ± 2 |
| G_0.1_Sr-HA | 16.5 ± 1 | 27 ± 0.6 | 36.5 ± 0.8 | 44 ± 1 | 52.8 ± 0.5 | dissolved | dissolved |
| G_0.05/G_0.2 | 22 ± 1 | 39 ± 1 | 44 ± 1 | 50 ± 2 | 54 ± 2 | 60 ± 2 | dissolved |
| G_0.1/G_0.2 | 14.7 ± 0.8 | 24 ± 1 | 34 ± 2 | 42 ± 1 | 49 ± 1 | 61 ± 1 | dissolved |
| G_0.15/G_0.2 | 11 ± 0.6 | 20 ± 0.8 | 25 ± 0.8 | 34 ± 1 | 42 ± 2 | 50 ± 2 | 65 ± 2 |
| G_0.1_Sr-HA/G_0.2 | 15 ± 1 | 24.3 ± 0.6 | 33 ± 1.4 | 40.8 ± 2 | 48.3 ± 1 | 61.3 ± 1 | dissolved |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, M.F.; Amadori, S.; Casolari, S.; Bigi, A.; Dolci, L.S.; Panzavolta, S. Cylindrical Layered Bone Scaffolds with Anisotropic Mechanical Properties as Potential Drug Delivery Systems. Molecules 2019, 24, 1931. https://doi.org/10.3390/molecules24101931
Di Filippo MF, Amadori S, Casolari S, Bigi A, Dolci LS, Panzavolta S. Cylindrical Layered Bone Scaffolds with Anisotropic Mechanical Properties as Potential Drug Delivery Systems. Molecules. 2019; 24(10):1931. https://doi.org/10.3390/molecules24101931
Chicago/Turabian StyleDi Filippo, Maria Francesca, Sofia Amadori, Sonia Casolari, Adriana Bigi, Luisa Stella Dolci, and Silvia Panzavolta. 2019. "Cylindrical Layered Bone Scaffolds with Anisotropic Mechanical Properties as Potential Drug Delivery Systems" Molecules 24, no. 10: 1931. https://doi.org/10.3390/molecules24101931