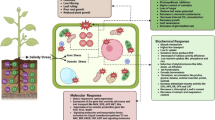
Abstract
The objective of this study was to determine more indepth physiological and antioxidant responses in two Medicago ciliaris lines (a salt-tolerant line TNC 1.8 and a salt-sensitive line TNC 11.9) with contrasting responses to 100 mM NaCl. Under salt stress, both lines showed a decrease in total biomass and in the growth rate for roots, but TNC 1.8 was less affected by salt than TNC 11.9 in that it maintained leaf growth even in the presence of added salt. In both lines, salt stress mainly affected micronutrient status (Fe, Mn, Cu and Zn) rather than K nutrition, but the tolerant line TNC 1.8 accumulated more Na in leaves and less in roots compared with TNC 11.9. Salt stress decreased total soluble sugars (TSS) in all organs of the sensitive line TNC 11.9, whereas TSS was only reduced in roots of the tolerant line. The salt-induced drop in growth was linked to an increase in lipid peroxidation in roots of both lines and in leaves of the sensitive line. The salt-tolerant line TNC 1.8 was more efficient at managing salt-induced oxidative damage in leaves and to a lesser extent in roots than the salt-sensitive line TNC 11.9, by preserving higher phenolic compound and superoxide dismutase levels in both organs.
Similar content being viewed by others
Abbreviations
- CE:
-
catechin equivalent
- DM:
-
dry matter
- GAE:
-
gallic acid equivalents
- MDA:
-
malondialdehyde
- RGR:
-
relative growth rate
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- TBA:
-
thiobarbituric acid
- TCA:
-
trichloroacetic acid
- TSS:
-
total soluble sugars
References
Abdelly C., Debez A., Smaoui A. & Grignon C. 2011. Halophytefodder species association may improve nutrient availability and biomass production of the sabkha ecosystem, pp. 85–94. In: Öztürk M., Böer B., Barth J.H., Breckle S.W., Clüsener-Godt M. & Khan M.A. (eds), Sabkha Ecosystems, Volume 3: Africa and Southern Europe. Springer, Drodrecht, Heidelberg, London, New York.
Abdul Jaleel C., Gopi R., Sankar B., Manivannan P., Kishorekumar A., Sridharan R. & Panneerselvam R. 2007. Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. South Afr. J. Bot. 73: 190–195.
Ballesteros E., Blumwold E., Donnaire J.P. & Belver A. 1997. Na+/H+ antiport activity in tonoplast vesicles isolated from sunflower roots induced by NaCl stress. Physiol. Plant. 9: 328–334.
Bates L.S., Waldren R.P. & Teare I.D. 1973. Rapid determination of the free proline in water stress studies. Plant Soil 39: 205–208.
Ben Salah I., Albacete A., Martínez Andújar C., Haouala R., Labidi N., Zribi F., Martinez V., Pérez-Alfocea F. & Abdelly C. 2009. Response of nitrogen fixation in relation to nodule carbohydrate metabolism in Medicago ciliaris lines subjected to salt stress. J. Plant Physiol. 166: 477–488.
Ben Salah I., Slatim T., Gruber M., Mesedi D., Gandour M., Benzarti M., Maouala R., Zribi K., Ben Hamed K., Perez-Alfocea F. & Abdelly C. 2011. Relationship between symbiotic nitrogen fixation, sucrose synthesis, and antioxidant activities in source leaves of two Medicago ciliaris lines cultivated under salt stress. Environ. Exp. Bot. 70: 166–173.
Bhattacharjee S. & Mukherjee A.K. 1996. Ethylene evolution and membrane lipid peroxidation as indicators of salt injury in leaf tissues of Amaranthus lividus seedlings. Ind. J. Exp. Biol. 34: 279–281.
Bradford M. 1976. A rapid and sensitive method for the quantification of microgram quantities of proteins utilising the principal of protein-dye binding. Anal. Biochem. 72: 248–254.
Cavalcanti F.R., Lima J.P.M.S., Ferreira-Silva S.L., Viégas R.A. & Silveira J.A.G. 2007. Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J. Plant Physiol. 164: 591–600.
Chalker-Scott L. 1999. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70: 1–9.
Chanwitheesuk A., Teerawutgulrag A. & Rakariyatham N. 2005. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 92: 491–497.
Chinnusamy V., Jagendorf A. & Zhu J.K. 2005. Understanding and improving salt tolerance in plants. Crop Sci. 45: 437–448.
Debez A., Saadaoui D., Ramanib B., Ouerghi Z., Koyro H.W., Huchzermeyer B. & Abdelly C. 2006. Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ. Exp. Bot. 57: 285–295.
Dewanto V., Wu X., Adom K.K. & Liu R.H. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50: 3010–3014.
Di Baccio D., Navari-Izzo F. & Izzo R. 2004. Seawater irrigation: antioxidant defence responses in leaves and roots of a sunflower (Helianthus annuus L.) ecotype. J. Plant Physiol. 161: 1359–1366.
Dixon R.A. & Paiva N. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell 7: 1085–1097.
Gómez J.M., Hernandez J.A., Jimenez A., del Rio L.A. & Sevilla F. 1999. Differential response of antioxidative system of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Radic. Res. 31: 11–18.
Gossett D.R., Millhollon E.P. & Lucas M.C. 1994. Antioxidant responses to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 34: 706–714.
Gould K.S., Markham K.R., Smith R.H. & Goris J.J. 2000. Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 51: 1107–1115.
Grassmann J., Hippeli S. & Elstner E.F. 2002. Plant’s defence and its benefits for animals and medicine: role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiol. Biochem. 40: 471–478.
Hernandez J.A. & Almansa M.S. 2002. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant. 115: 251–257.
Hewitt E.J. 1966. Sand and Water Culture Methods Used in the Study of Plant Nutrition; 2nd Ed. Commonwealth Agricultural Bureau, Farnham Royal, 547 pp.
Kholová J., Sairam R.K., Meena R.C. & Srivastava G.C. 2009. Response of maize genotypes to salinity stress in relation to osmolytes and metal ions contents, oxidative stress and antioxidant enzymes activity. Biol. Plant. 53: 249–256.
Kjeldahl J.Z. 1983. New methode zyr Besimming des stickstoffs in organischen Körpen. Anal. Chem. 22: 366–382.
Koca H., Ozdemir F. & Turkan I. 2006. Effect of salt stress on lipid peroxidation and superoxide dismutase and peroxidase activities of Lycopersicon esculentum and L. pennellii. Biol. Plant. 50: 745–748.
Ksouri R., Megdiche W., Debez A., Falleh H., Grignon C. & Abdelly C. 2007. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 45: 244–249.
Lisiewska Z., Kmiecik W. & Korus A. 2006. Content of vitamin C, carotenoids, chlorophylls and polyphenols in green parts of dill (Anethum graveolens L.) depending on plant height. J. Food Compos. Anal. 19: 134–140.
Mäeser P., Gierth M. & Schroeder J.I. 2002. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil 247: 43–54.
Mengel K. & Kirkby E.A. 2001. Plant nutrients, pp. 1–14. In: Mengel K., Kirkby E.A., Kosegarten H. & Appel T. (eds) Principles of Plant Nutrition. Kluwer Academic Publishers, Dordrecht.
Mittova V., Tal M., Volokita M. & Guy M. 2003. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in responses to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 26: 845–856.
Munns R. & Tester M. 2008. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 59: 651–681.
Naczk M. & Shahidi F. 2004. Extraction and analysis of phenolics in food. J. Chromatogr. 1054: 95–111.
Navarro J.M., Flores P., Garrido C. & Martinez V. 2006. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem. 96: 66–73.
Noctor G. & Foyer C.H. 1998. Ascorbate and glutathione: keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 49: 249–279.
Oliva S.R., Raitio H. & Mingorance M.D. 2003. Comparison of two wet digestion procedures for multi-element analysis of plant samples. Commun. Soil Sci. Plant Anal. 34: 2913–2923.
Polle A. & Rennenberg H. 1993. Significance of antioxidants in plant adaptation to environmental stress, pp. 263–273. In: Fowden L., Mansfield T. & Stoddart J. (eds), Plant Adaptation to Environmental Stress. Chapman & Hall, London.
Poustini K., Siosemardeh A. & Ranjbar M. 2007. Proline accumulation as a response to salt stress in 30 wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Gen. Resour. Crop Evol. 54: 925–934.
Scebba F., Sebastiani L. & Vitagliano C. 1999. Protective enzymes against activated oxygen species in wheat (Triticum aestivum L.) seedlings: responses to cold acclimation. J. Plant Physiol. 55: 762–768.
Sgherri C., Stevanovic B. & Navari-Izzo F. 2004. Role of phenolics in the antioxidative status of the resurrection plant Ramonda serbica during dehydration and rehydration. Physiol. Plant. 122: 478–488.
Shevyakova N.I., Bakulina E.A. & Kuznetsov V.I.V. 2009. Proline antioxidant role in the common ice plant subjected to salinity and paraquat treatment inducing oxidative stress. Russ. J. Plant Physiol. 56: 663–669.
Smirnoff N., Conklin P.L. & Loewus F.A. 2001. Biosynthesis of ascorbic acid in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 52: 437–467.
Srinivas N.D., Rshami K.R. & Raghavarao K.S.M.S. 1999. Extraction and purification of a plant peroxidase by aqueous two-phase extraction coupled with gel filtration. Process Biochem. 35: 43–48.
Sun B., Ricardo-da-Silva J.M. & Spranger I. 1998. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 46: 4267–4274.
Tester M. & Davenport R. 2003. Na+ tolerance and Na+ transport in higher plants. Annals Bot. 91: 503–527.
Tounekti T., Vadel A.M., Onate M., Khemira H., Munné-Bosch S. 2011. Salt-induced oxidative stress in rosemary plants: damage or protection? Environ. Exp. Bot. 71: 298–305.
Türkan I. & Demiral T. 2009. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 67: 2–6.
Verbruggen N. & Hermans C. 2008. Proline accumulation in plants: a review. Amino Acids 35: 753–759.
Wang X.S. & Han J.G. 2009. Changes of proline content, activity, and active isoforms of antioxidative enzymes in two alfalfa cultivars under salt stress. Agric. Sci. China 8: 431–440.
Yasici I., Türkan I., Sekmen A.H. & Demiral T. 2007. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 61: 49–57.
Yemm E.W. & Willis J. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57: 508–514.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Salah, I.B., Mahmoudi, H., Gruber, M. et al. Phenolic content and antioxidant activity in two contrasting Medicago ciliaris lines cultivated under salt stress. Biologia 66, 813–820 (2011). https://doi.org/10.2478/s11756-011-0102-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-011-0102-6