Abstract
Keywords
Programmed cell death Prostate cancer Curcumin Nanoparticles PLGA
Introduction
Prostate cancer is the most frequently diagnosed cancer and the leading cause of cancer deaths among males worldwide. The best lines of defense, radiation therapy, and chemotherapy are unsatisfactory due to the untoward side effects on healthy cell and the problem of drug resistance (1-4). Searching for new compounds for the treatment of cancer is the aim of numerous studies, and many works are focused on plant-derived compounds that have curative potential. Curcumin (Cur) is a phenolic compound with yellow color from the plant, Curcuma longa, which has been used as a kind of traditional Asian medicine for centuries (1, 5).
Cur has many beneficial effects including anti-inflammatory, antioxidant, anticancer, antimicrobial, and anti-hyperlipidemic (6-10). Cur has also been shown to possess potent anti-neoplastic activity against a number of tumors including prostate, breast and colon cancer (11, 12). However, lack of aqueous solubility, rapid metabolism and conjugation in the liver has limited systemic bioavailability of Cur (13). Cur is lipophilic in nature. When nanoparticles of Cur is formulated with some amphiphilic polymer or phospholipids, Cur partitions and gets encapsulates into the hydrophobic core of nanoparticles which not only enhance its bioavailability but also increase its stability by protecting them from the influence of metabolizing enzymes (14).
Polymers can be modified to have altered surface properties of the formulated nanoparticles. Different functional groups can be covalently or non-covalently conjugated with the polymeric chains to increase the mean residence time of the nanoparticles in the gastrointestinal mucosa (15). The advantages of such formulations are their low toxicity, high stability providing longer circulation and smaller size which attribute them increased cellular permeability for passive targeting of solid tumor tissue site with enhanced permeation and retention effect (16). It has been revealed that nanoparticle encapsulation improves oral bioavailability of Cur up to 9-folds compared to that of free Cur (17). Poly lactic-co-glycolic acid (PLGA) is extensively utilized for the development of polymeric nanoparticle of Cur (18). Xie et al. have shown that PLGA nanoparticles improve the oral bioavailability of Cur in rats (19). Yallapu et al. have shown that Cur loaded PLGA can inhibit proliferation of prostate cancer cells (18).
Defects in cancer cell death are the most frequent causes of therapeutic resistance, and thus exploring cancer cell death might inform development of strategies to overcome therapeutic resistance (20). In the present study, anticancer effects of Cur encapsulated in PLGA (NCur) on PC3 prostatic cancer cell line were investigated by assessment of cell viability, apoptosis and autophagy.
Experimental
Materials
Poly (d,l-lactic-co-glycolic acid) (PLGA) with co-polymerization ratio 50:50 (lactic/glycolic), Polyvinyl alcohol (PVA) Mw ≈ 130,000, curcumin, 3-(4, 5dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and DAPI (4′, 6-diamidino-2-phenylindole) were purchased from Sigma (Steinheim, Germany). Dulbecco’s Modified Eagel Medium (DMEM), Chloroform, ethanol, fetal bovine serum and Annexin V-FITC/propidium iodide assay kit were obtained from Gibco, Invitrogen (Carlsbad, CA, USA). Primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of NCur
Curcumin loaded PLGA nanoparticles were prepared by solvent (s/o/w) evaporation technique. Briefly, 60 mg of the PLGA were dissolved in 1 mL chloroform as an oil phase (organic solution). Free Cur (6 mg) was added to the PLGA/chloroform solution and sonicated to produce the s/o primary emulsion. To form the final s/o/w emulsion this emulsion was then added to a solution of 2% PVA and ethanol (1:1) and again was sonicated at 55 W for 2 min. To evaporation (remove the organic phase) of solvent (chloroform) s/o/w emulsion was sonicated and agitated by stirrer for 5-6 h. The sample was then centrifuged at 15000×g for 10 min and washed 2-3 times with distilled water. It was then freeze dried for 24 h to obtain the dry powder. The nanoparticles were stored at 4 °C for further use (16, 21).
Characterization of NCur
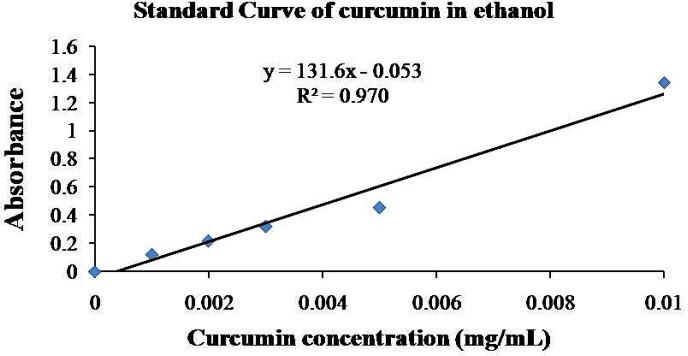
For characterization of NCur, encapsulation efficiency and particle size of the nanoparticles were determined. The encapsulation efficiency of the nanospheres was determined by analyzing the supernatant of the final emulsion once the nanospheres were removed from it by centrifugation at 15000×g for 15 min. For the estimation of Cur present in the supernatant, the absorbance was measured spectrophotometrically at 425 nm and the amount of drug present was calculated from calibration curves of concentration versus absorbance with known standards of the drug. Encapsulation efficiency (EE %) and Cur loading were calculated using below formula (16, 21).
Amount of Cur in the nanoparticles = Total amount of Cur- free Cur
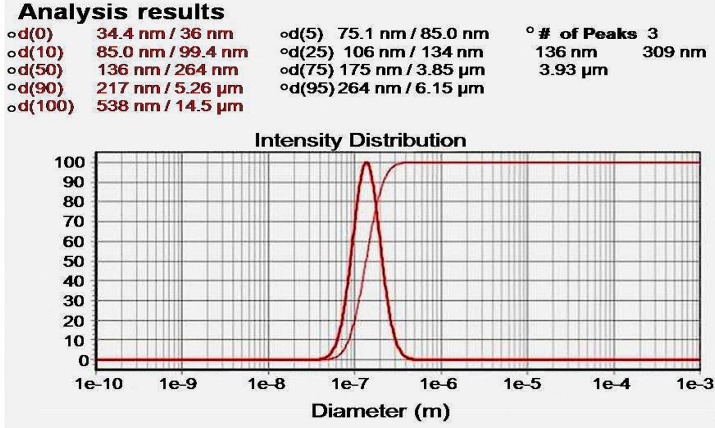
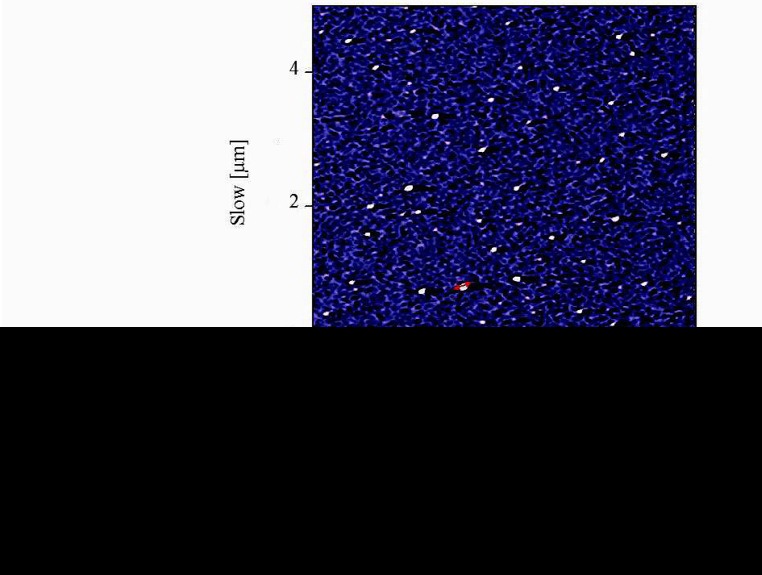
Atomic force microscopy (AFM) method was used to determine the size and morphology of the synthesized NCur.
Experimental design
The human PC3 cell line was purchased from Pasteur Institute of Iran. PNT2 cell line (Normal human prostate epithelium) was purchased from sigma (Cat number: 95012613). Control group was untreated cells. Experimental groups were treated by 25 μg/mL of Cur and 25 μg/mL of NCur for 48 h, respectively. The dose of NCur was selected based on the results of previous studies (16) and our pilot study. Briefly, before the experiment, we examined different doses of NCur (1, 5, 25 and 50 µg/mL) for 24 and 48 h to determine the best one for anticancer action and 3 flasks were used for each dose. By using MTT assay, the percentage of viable cells was significantly decreased at the concentration of 25 and 50 µg/mL (results not shown). There was no significant difference between concentration of 25 and 50 µg/mL. Thus, we used Cur and NCur at the dose of 25 µg/mL in this study. Prior to beginning each test, manual cell counting by using a Hemacytometer was performed in order to standardize cell concentration between samples to minimize error and variation in downstream results (22).
MTT assay
MTT(3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium-bromide) assays were used to compare the effect of NCur with Cur on cell viability and proliferation as previously described (23). Briefly, PC3 and PNT2 cells were seeded in 96-well plates (1 × 104 cells/well). After treatment, the cells were maintained with culture media for 48 h. MTT (0.5 mg/mL) was then added to each well, and cells were further incubated for 4 h at 37 °C. Supernatants were then removed, and 700 µL of DMSO were added to each well to dissolve the formazan product. Absorbance at 540 nm was measured using a microplate reader (BioRad, Hercules, CA). Optical density values of the control cells were calculated as 100% viability. Because absorbance is in proportion to the number of living cells in a sample, the MTT assay reflects the extent of cell proliferation (24).
DAPI staining
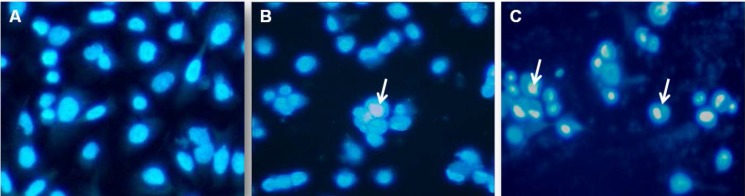
The PC3 and PNT2 cells (104 cells/mL) were grown on cover slips after treatment with NCur or Cur for 48 h. Nuclei changes of apoptosis were assayed by DAPI (4,6-diamidino-2-phenylindole) staining as previously described (25). Following washed with PBS, the cells were fixed with cold methanol/acetone for 5 min at room temperature, washed with PBS, and then incubated with DAPI solution (2 mg/mL in PBS) for 10 min at room temperature. Fluorescence images were captured using a fluorescence microscope (Olympus, Japan).
Immunocytochemistry
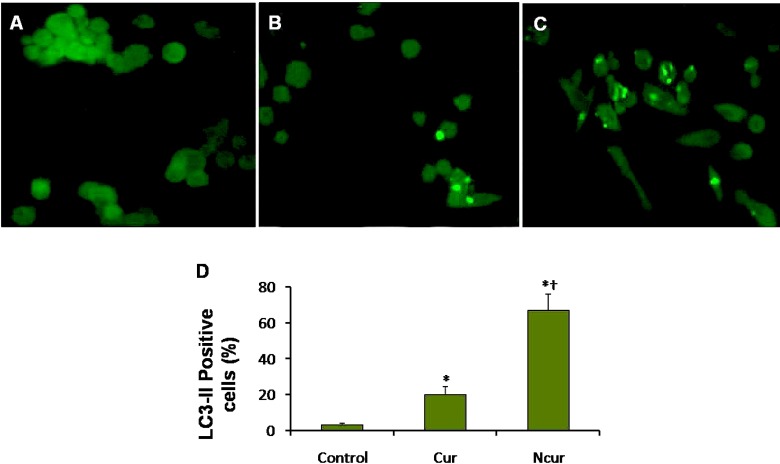
The PC3 and PNT2 cells (104 cells/ mL) after treatment deposited on a glass cover slip were fixed for 15 min in 4% paraformaldehyde in PBS at 4 °C. Nonspecific binding was blocked with 1% bovine serum albumin-PBS for 15 min. Anti-LC3-II antibody (sc-16755, Santa Cruz) at 1/100 dilution was applied overnight at 4 °C. After two washes with PBS, the sections were incubated with FITC-conjugated anti-mouse secondary antibody (sc-2356, Santa Cruz) for 1 h at room temperature. After washing, the cells were incubated with DAPI at dilution 1: 1000 in PBS for 10 min. Fluorescence images were captured using a fluorescence microscope (Olympus, Japan). The percentage of LC3-II positive cells was calculated by dividing the number of LC3-II positive cells in a randomly microscopy field by the total number of cells in that field, and the result was multiplied by 100. There were at least 3 slides for different groups. Ten randomly field were evaluated for each slide (26).

MTT assay of PNT2 cells in various groups. Percentage of cell viability in 24 and 48 h are presented
| Groups | 24 h | 48 h |
|---|---|---|
| Control | 100 ± 0.01 | 100 ± 0.02 |
| Cur | 98.1 ± 0.35 | 100.3 ± 1.32 |
| NCur | 99.4 ± 0.35 | 101.2 ± 1.65 |
Annexin V-FITC/propidium iodide apoptosis assay
PC3 and PNT2 cells were placed in a six-well culture plate and treated with Cur or NCur for 48 h as experimental groups. Control group was untreated cells. Normal, apoptotic and necrotic cells were distinguished using an Annexin V-FITC/propidium iodide assay kit according to the manufacturer’s instructions. Briefly, after the incubation period, the cells (1.0 × 106) were washed in cold PBS. The washed cells were re-centrifuged, discarded the supernatant, and resuspended in 1X annexin-binding buffer. Twenty five μL of the Annexin V conjugate and 2 μL of the 100 μg/mL propidium iodide (PI) working solution were added to each 100 μL of cell suspension. The cells were incubated at room temperature for 15 min and then were washed with 1X Annexin-Binding buffer and deposited onto slides.
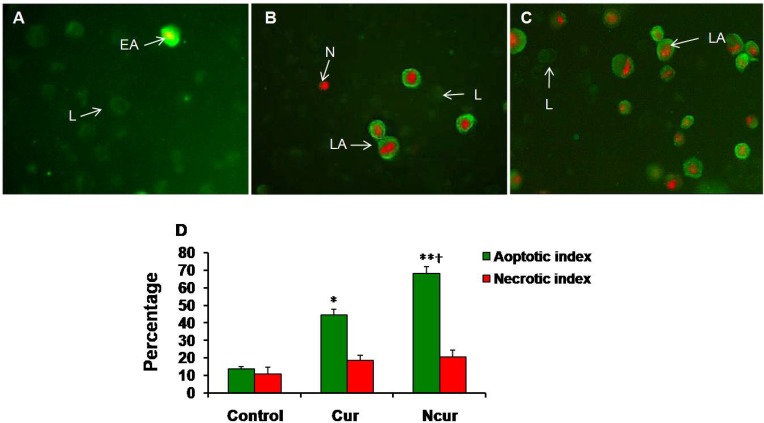
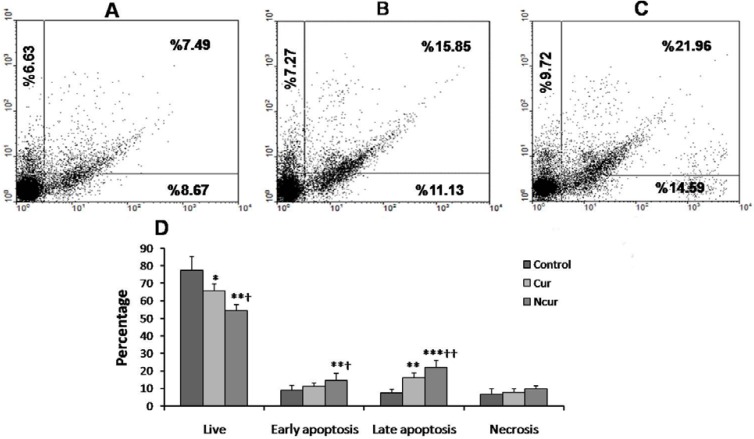
The fluorescence was observed by using appropriate filters. The cells were separated into three groups: live, apoptotic (early and late), and necrotic cells. Live cells showed only weak Annexin V staining of the cellular membrane. Early apoptotic cells showed a significantly higher degree of surface labeling. Late apoptotic cells showed both membrane staining by Annexin V and strong nuclear staining from the PI. Necrotic cells showed only nuclear staining from the PI. Apoptotic index and necrotic index were calculated by dividing the number of apoptotic or necrotic cells in a randomly microscopy field by the total number of cells in that field, and the result was multiplied by 100. The Apoptotic indexes and necrotic indexes of 10 randomly field were evaluated and the mean apoptotic index and necroptotic index of each case were calculated (24). Flow cytometry by using this kit was also performed. Briefly, the cells were exposed to Cur or NCur in 6-well plates for 48 h. After exposure, cells were trypsinized and centrifuged at 1000 rpm, the cell pellet was washed with PBS once and re-suspended in 100 mL of binding buffer, then incubated with 2 mL Annexin V-FITC for 10 min, which was followed by staining with 2 mL PI. Then, the samples were diluted with 400 mL binding buffer and analyzed with a Flow cytometer (Becton Dickinson, San Jose, CA), and at least 10000 cells were counted for each sample. The cell population of interest was gated on the basis of the forward and side-scatter properties. The different labeling patterns in the Annexin V/PI analysis identified the different cell populations where FITC negative and PI negative were designated as viable cells; FITC positive and PI negative as early apoptotic cells; FITC positive and PI positive as late apoptotic cells and FITC negative and PI positive as necrotic cells. The data analysis was performed using WinMDI 2.9 software.
Data Analysis
Comparisons of multiple (> 3) group means were performed using one-way analyses of variance and post-hoc procedures based on Newman-Keuls tests. Student’s t tests were used for comparisons of two group means. A p-value less than 0.05 is considered statistically significant.
Results
Characterization of NCur
The particle size distribution showed a range of 100 nm to 200 nm, with the mean particle size being 136 nm (Figure 1). The encapsulation efficiency of Cur-loaded PLGA nanospheres was 97 ± 0.45%. Standard curve is shown in Figure 2. AFM revealed the size and morphology of the synthesized NCur. The complexes appear spherical with a mean size between 100 and 200 nm as can be seen in Figure 3. In addition, the NCur-loaded PLGA nanospheres prepared were completely dispersed in aqueous media with no aggregates as opposed to free Cur which exhibits poor aqueous solubility (Figure 4).
Cell viability and proliferation
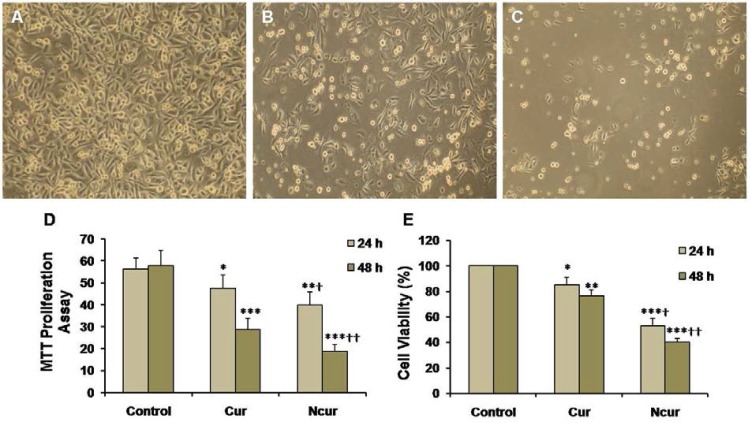
MTT assays showed that exposure of PC3 to Cur induced a significant decrease in cell viability (p < 0.01). Cell viability in the NCur treated cells were significantly decreased compared to Cur treatment (p < 0.05). Viability of PNT2 cells were not changed in response to Cur or NCur (Table 1). MTT proliferation assay also showed that NCur significantly inhibited growth of PC3 cells in comparison of Cur. These results are shown in Figure 5.
DAPI staining
In order to further elucidate NCur cell death in PC3 cells, we analyzed cell nuclear morphology with DAPI staining after 48 h of Cur or NCur treatment. As shown in Figure 6 NCur-treated PC3 cells exhibited chromatin condensation which indicated cell apoptosis. Apoptotic index in PNT2 cells was not significantly changed in response to Cur or Ncur (the results not shown).
Annexin V-FITC/propidium iodide apoptosis assay
A few necrotic and apoptotic cells were observed in control group by using a fluorescence microscope. In Cur-treated cells, a large number of PC3 cells showed apoptosis. In NCur- treated cells, the percentage of apoptotic cells (apoptotic index) was significantly increased in comparison to free Cur group (p < 0.01). There were not significant changes in necrotic index between control and experimental groups. Similar results were obtained by using Flow cytometry method. These results are shown in Figures 7 and 8. Apoptotic and necrotic index in PNT2 cells were not significantly changed in response to Cur or Ncur (the results not shown).
Autophagy
Autophagy was assessed by using immunocytochemistry for LC3-II and AO staining for detect autophagosomes. Autophagosomes (LC3 positive vesicles) showed light green appearance. A few LC3 positive cells were observed in control group. The percentage of LC3-II positive cells in Cur treatment was significantly increased. The percentage of LC3-II positive cells in NCur treatment was significantly increased in compared to Cur treated cells. These results are shown in Figure 9. In PNT2 cells, the percentage of LC3-II positive cells was not significantly changed in response to Cur or Ncur (the results not shown).
Discussion
This study demonstrated that encapsulation of Cur in PLGA could effectively enhance its anticancer effects. NCur could effectively inhibit the growth of PC3 cells and induced apoptosis and autophagy in these cells. NCur could reduce viability of PC3 cells. Nair et al. showed that cellular uptake of Cur encapsulated in PLGA in human epithelial cervical cancer cells (HeLa) were enhanced compared to free Cur. They have also proved that NCur have more pronounced antitumor activity by using anti-proliferative studies (MTT assay) and Annexin V/propidium iodide staining (16). Mukerjee et al. by using cell viability studies have demonstrated that Cur encapsulated in PLGA is able to exert a more pronounced effect on the prostate cancer cells as compared to free Cur (21).
Other studies of Cur formulations such as micellar aggregates of cross-linked and random copolymers of N-isopropylacrylamide, with N-vinyl-2- pyrrolidone and poly (ethyleneglycol)
monoacrylate and self-assembling methoxy poly(ethylene glycol)–palmitate Cur nanocarrier have shown to exhibit similar growth inhibition to that of free Cur (27-29).
A cationic poly (vinyl pyrrolidone) -Cur conjugate has been judged by MTT assay to be more potent in L929 fibroblast cells over free Cur (30). Tang et al. have demonstrated that polycatocol-Cur conjugate is highly cytotoxic to ovarian cancers (SKOV-3 and OVCAR-3) and MCF-7 breast cancer cell lines (31).
As shown in results, NCur can effectively increase apoptosis in PC3 cells. Most current anticancer drugs kill actively dividing cells by the induction of apoptosis (32). Apoptotic cell death involves a series of events leading to characteristic changes in cell morphology, including loss of cell membrane asymmetry, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation, and activation of caspases (33). In DAPI staining we observed that NCur considerably induced chromatin condensation and nuclear fragmentation. The results of annexin V/PI assay revealed that apoptosis, not necrosis, was the predominant mechanism in NCur-induced cytotoxicity.
Unfortunately, cancer cells often acquire resistance to agents that activate the apoptotic pathway (36). Thus activation of other death pathways may be helpful to management of cancer therapy. Autophagy has recently gained much attention for its paradoxical roles in cell survival and cell death, particularly in the pathogenesis as well as the treatment of cancer (34, 35). Whether autophagy enables cells to survive or enhances their death is context-driven, depending on the type of stimuli, nutrient availability, organism development, and apoptotic status (36).
Autophagy induced during starvation, growth factor deprivation, hypoxia, endoplasmic reticulum stress, and microbial infection can prevent cell death (37). However, it can be also associated with cell death due to excessive mitophagy, leading to loss of mitochondrial membrane potential (Δψm), caspase activation, and lysosomal membrane permeabilization (38).
As shown in results, NCur can effectively increase percentage of LC3-II positive PC3 cells. During autophagosome formation, cytosolic microtubule-associated protein light chain 3-I (LC3-I) is conjugated with phosphatidylethanolamine and converted to LC3-II. This phosphatidylethanolamine-conjugated LC3-II, detectable by immunoblotting, is present specifically on isolation membranes and autophagosomes and therefore serves a second and widely accepted approach to monitoring autophagia (39).
During autophagy, parts of the cytoplasm are digested by lysosomes, thereby providing metabolites that are used for cell homeostasis. Although autophagy is a process with a major role in cell survival, it is also capable of inducing cell death characterized by extensive digestion of intracellular organelles (40). Importantly, a number of small molecules (including several anticancer drugs) activate autophagy both in-vivo and in-vitro in cancer cells, indicating the importance of this biological process in the development of chemotherapeutics (41, 42). The importance of autophagic cell death induced by some anticancer therapeutic agents in relation to the regression of cancer cell growth through multiple mechanisms is recently emerging. For example the cytotoxic compound docosahexaenoic acid can induce both apoptosis and autophagic cell death (43).
The exact mechanism of NCur effects on PC3 cells is not obtained from this study. Our results have demonstrated that NCur enhances both apoptosis and autophagia processes in PC3 cells. Additionally, viability of PC3 cells was considerably reduced by NCur. These findings indicate that NCur can induce both type I and II programmed cell death.
Mouratidis et al. showed that Lupulone, a β-acid derived from hop extracts, induced apoptosis and autophagia in PC3 and DU145 prostate cancer cells (44). Ullén et al. have also demonstrated that sorafenib reduces cell viability and induces apoptosis and cellular autophagy (45). In cancer, an autophagy paradox has emerged in which survival and death are context specific, particularly due to complex interactions between autophagic and apoptotic pathways. Accordingly, cancer therapies have been reported to have opposing effects on cell death. Photodynamic therapy promotes autophagic cell death in apoptosis-deficient cancer cells (46), whereas sulforaphane-induced autophagy in PC3 and LNCaP cells is protective (47). Furthermore, manipulation of autophagy can sensitize tumor cells to subsequent treatments. Induction of autophagy by an mTOR inhibitor increased prostate cancer cell susceptibility to irradiation (48).
Additionally, excessive autophagy will also inevitably trigger autophagic cell death or type II programmed cell death. Due to emerging links between autophagic aberrations and cancer, there is rapidly growing support that this type II programmed cell death may also function as a suppressor of tumorigenesis (40).
Conclusion
In summary, this study has demonstrated that NCur stimulates the both type I and II programmed cell death and appear to be good candidates for further preclinical studies of prostate cancer treatment. Extrapolation of these data to the human situation is not appropriate. However, this information does provide a stimulus for true clinical investigations.
Acknowledgements
References
-
1.
Shi M, Cai Q, Yao L, Mao Y, Ming Y, Ouyang G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell. Biol. Int. 2006;30:221-6. [PubMed ID: 16376585].
-
2.
Gonzalo ML, Isaacs WB. Molecular pathways to prostate cancer. J. Urol. 2003;170:2444-52. [PubMed ID: 14634448].
-
3.
Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2007;12:20-37. [PubMed ID: 17227898].
-
4.
Richie JP. Anti-androgens and other hormonal therapies for prostate cancer. Urology. 1999;54:15-18. [PubMed ID: 10606279].
-
5.
Walters DK, Muff R, Langsam B, Born W, Fuchs B. Cytotoxic effects of curcumin on osteosarcoma cell lines. Invest. New Drugs. 2008;26:289-97. [PubMed ID: 18071634].
-
6.
Jacob A, Wu R, Zhou M, Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Res. 2007:89369. [PubMed ID: 18274631].
-
7.
Kumar P, Kumar A. Possible role of sertraline against 3-nitropropionic acid induced behavioral, oxidative stress and mitochondrial dysfunctions in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:100-8. [PubMed ID: 19022325].
-
8.
Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008;32:1119-23. [PubMed ID: 18425340].
-
9.
Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem. Pharmacol. 2008;75:787-809. [PubMed ID: 17900536].
-
10.
Arafa HM. Curcumin attenuates diet-induced hypercholesterolemia in rats. Med. Sci. Monit. 2005;11:228-34.
-
11.
Sharma RA, Gescher AJ, Steward W. Curcumin: the story so far. Eur. J. Cancer. 2005;41:1955-68. [PubMed ID: 16081279].
-
12.
Aggarwal BB, Kumar A, Bharti A. Anti cancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:3633-98.
-
13.
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807-18. [PubMed ID: 17999464].
-
14.
Das MK, Chakraborty T. Curcumin nano-therapeutics for cancer chemotherapy: promises and challenges for the future. Eur. J. Pharm. Med. Res. 2016;3:177-91.
-
15.
Grabovac V, Bernkop-Schnürch A. Development and in-vitro evaluation of surface modified poly (lactide-co-glycolide) nanoparticles with chitosan-4-thiobutylamidine. Drug Dev. Ind. Pharm. 2007;33:767-74. [PubMed ID: 17654025].
-
16.
Nair KL, Thulasidasan AK, Deepa G, Anto RJ, Kumar GS. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int. J. Pharm. 2012;425:44-52. [PubMed ID: 22266528].
-
17.
Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MNV. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009;37:223-30. [PubMed ID: 19491009].
-
18.
Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N, Ganju A, Balakrishna S, Gupta BK, Zafar N, Jaggi M, Chauhan SC. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35:8635-48. [PubMed ID: 25028336].
-
19.
Xie X, Tao Q, Zou Y, Zhang F, Guo M, Wang Y, Wang H, Zhou Q, Yu S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J. Agric. Food Chem. 2011;59:9280-89. [PubMed ID: 21797282].
-
20.
Ojha R, Ishaq M, Singh SK. Caspase-mediated crosstalk between autophagy and apoptosis: mutual adjustment or matter of dominance. J. Cancer Res. Ther. 2015;11:514-24. [PubMed ID: 26458576].
-
21.
Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867-75. [PubMed ID: 19846921].
-
22.
Cadena-Herrera D, E Esparza-De Lara J, D Ramírez-Ibañez N, A López-Morales C, O Pérez N, F Flores-Ortiz L, Medina-Rivero E. Validation of three viable-cell counting methods: Manual, semi-automated, and automated. Biotechnology Reports. 2015;7:9-16. [PubMed ID: 28626709].
-
23.
Ding Y, Gao H, Zhang Y, Li Y, Vasdev N, Gao Y, Chen Y, Zhang Q. Alantolactone selectively ablates acute myeloid leukemia stem and progenitor cells. J. Hematol. Oncol. 2016;9:93. [PubMed ID: 27658462].
-
24.
Orazizadeh M, Khodadadi A, Bayati V, Saremy S, Farasat M, Khorsandi L. In-vitro toxic effects of zinc oxide nanoparticles on rat adipose tissue-derived mesenchymal stem cells. Cell J. 2015;17:412-21. [PubMed ID: 26464812].
-
25.
Rahbar Saadat Y, Saeidi N, Zununi Vahed S, Barzegari A, Barar J. An update to DNA ladder assay for apoptosis detection. Bioimpacts. 2015;5:25-8. [PubMed ID: 25901294].
-
26.
Khorsandi L, Nejad-Dehbashi F, Ahangarpour A, Hashemitabar M. Three-dimensional differentiation of bone marrow-derived mesenchymal stem cells into insulin-producing cells. Tissue Cell. 2015;47:66-72. [PubMed ID: 25554603].
-
27.
Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A. Polymeric nanoparticle-encapsulated curcumin (‘nanocurcumin’): a novel strategy for human cancer therapy. J. Nanobiotechnology. 2007;5:3. [PubMed ID: 17439648].
-
28.
Sahu A, Bora U, Kasoju N, Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly (ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008;4:1752-61. [PubMed ID: 18524701].
-
29.
Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly (ethylene oxide)-b-poly(e-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. A. 2008;86:300-10. [PubMed ID: 17957721].
-
30.
Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J. Pharm. Sci. 2011;100:504-11. [PubMed ID: 20848656].
-
31.
Tang H, Murphy CJ, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, Radosz M. Curcumin polymers as anticancer conjugates. Biomaterials. 2010;31:7139-49. [PubMed ID: 20591475].
-
32.
Fulda S. Tumor resistance to apoptosis. Int. J. Cancer. 2009;124:511-5. [PubMed ID: 19003982].
-
33.
Dos Anjos DO, Sobral Alves ES, Gonçalves VT, Fontes SS, Nogueira ML, Suarez-Fontes AM, Neves da Costa JB, Rios-Santos F, Vannier-Santos MA. Effects of a novel β-lapachone derivative on Trypanosoma cruzi: parasite death involving apoptosis, autophagy and necrosis. Int. J. Parasitol. Drugs. 2016;6:207-19.
-
34.
Ricci MS, Zong WX. Chemotherapeutic approaches for targeting cell death pathways. Oncologist. 2006;11:342-57. [PubMed ID: 16614230].
-
35.
Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat. Rev. Cancer. 2007;7:961-7. [PubMed ID: 17972889].
-
36.
Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 2007;13:7271-9. [PubMed ID: 18094407].
-
37.
Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung HJ. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69:700-8. [PubMed ID: 19147587].
-
38.
Kourtis N, Tavernarakis N. Autophagy and cell death in model organisms. Cell Death Differ. 2009;13:21-30.
-
39.
Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 2005;13:886-97.
-
40.
Roca H, Varsos Z, Pienta KJ. CCL2 protects prostate cancer PC3 cells from autophagic death via phosphatidylinositol 3-Kinase/AKT-dependent survivin up-regulation. J. Biol. Chem. 2008;283:25057-73. [PubMed ID: 18611860].
-
41.
Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R. Programmed cell death (PCD) Apoptosis, autophagic PCD, or others? Ann. N. Y. Acad. Sci. 2000;926:1-12.
-
42.
Lefranc F, Facchini V, Kiss R. Proautophagic drugs: a novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395-403. [PubMed ID: 18165616].
-
43.
Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J. Pharmacol. Exp. Ther. 2010;333:454-64. [PubMed ID: 20179157].
-
44.
Mouratidis PX, Colston KW, Tucknott ML, Tyrrell E, Pirianov G. An investigation into the anticancer effects and mechanism of action of hop β-acid lupulone and its natural and synthetic derivatives in prostate cancer cells. Nutr. Cancer. 2013;65:1086-92. [PubMed ID: 24168111].
-
45.
Ullen A, Farnebo M, Thyrell L, Mahmoudi S, Kharaziha P, Lennartsson L, Grander D, Panaretakis T, Nilsson S. Sorafenib induces apoptosis and autophagy in prostate cancer cells in-vitro. Int. J. Oncol. 2010;37:15-20. [PubMed ID: 20514392].
-
46.
Xue LY, Chiu SM, Azizuddin K, Joseph S, Oleinick NL. Protection by Bcl-2 against apoptotic but not autophagic cell death after photodynamic therapy. Autophagy. 2008;4:125-7. [PubMed ID: 18025862].
-
47.
Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828-35. [PubMed ID: 16740722].
-
48.
Jing K, Song KS, Shin S. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy. 2011;7:1348-58. [PubMed ID: 21811093].