Abstract
Background: Topical calcipotriene is frequently prescribed for the treatment of plaque-type psoriasis. Calcipotriene is currently available in the US as an ointment, a solution, a cream, and in a fixed-dose combination ointment with betamethasone dipropionate. Calcipotriene 0.005% has recently been formulated as a foam using a novel aqueous-based formulation to provide a new topical treatment option for patients with psoriasis.
Objective: The objective of this study was to evaluate the efficacy and safety of topical calcipotriene 0.005% foam for the treatment of mild to moderate plaque-type psoriasis.
Design: Two identical, randomized, double-blind, vehicle-controlled, 8-week phase III clinical trials.
Intervention: Subjects with plaque-type psoriasis affecting 2–20% of the body surface area, with an identifiable target lesion affecting the trunk or extremities, were randomized in a 2:1 ratio to calcipotriene foam (n=437) or vehicle foam (n= 222). Study medication was applied twice daily for 8 weeks.
Outcome Measures: Treatment success was defined as a score of 0 or 1 (clear or almost clear) on the Investigator’s Static Global Assessment (ISGA) psoriasis rating scale and a minimum improvement of ISGA score of at least 2 grades from baseline. Predefined target lesions were assessed for erythema, scaling, and plaque thickness. Primary endpoint was the proportion of subjects in each treatment group who achieved treatment success after 8 weeks, analyzed on an intent-to-treat (ITT) basis. In the primary endpoint analysis, subjects missing 8-week outcomes data were classified as treatment failures regardless of their outcomes at earlier evaluations. As part of the sensitivity analysis, a last-observation-carried-forward (LOCF) approach to impute missing 8-week efficacy outcomes also examined treatment. Secondary endpoints included treatment success as a function of baseline ISGA score (mild or moderate), ISGA score of 0 or 1 (clear or almost clear), and effects of treatment on target lesion. Adverse events (AEs) were recorded throughout the study.
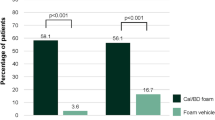
Results: In the ITT population of Study 1, treatment success after 8 weeks was achieved by 14% of subjects in the calcipotriene foam group versus 7% of subjects in the vehicle foam group (p =0.058). In the LOCF analysis, treatment success was achieved by more subjects with calcipotriene foam than with vehicle foam (15% vs 7%; p = 0.034). In Study 2, treatment success was achieved by more subjects in the calcipotriene foam group for the primary endpoint (27% vs 16%; p = 0.016) and the LOCF analysis (28% vs 16%; p = 0.010). Subjects in the calcipotriene foam group exhibited better response rates than did the vehicle foam group for most of the secondary outcomes. Calcipotriene foam was safe with an overall incidence of AEs similar to those experienced in the vehicle foam group. Application-site reactions were noted in approximately 1–2% of subjects in each group. No AE was reported in more than 2% of subjects in the calcipotriene foam group. Treatment was discontinued because of AEs in approximately 2% of subjects in both groups.
Conclusions: In two identically designed, phase III clinical trials, calcipotriene 0.005% foam was safe and effective for the treatment of mild to moderate plaque-type psoriasis for up to 8 weeks.
Clinical Trial Registration: Registered at clinicaltrials.gov: NCT00688519 and NCT00689481.








Similar content being viewed by others
References
Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol 2009; 60: 218–24
Del Rosso J, Friedlander SF. Corticosteroids: options in the era of steroid-sparing therapy. J Am Acad Dermatol 2005; 53 (1 Suppl. 1): S50–8
Pearce DJ, Stealey KH, Balkrishnan R, et al. Psoriasis treatment in the United States at the end of the 20th century. Int J Dermatol 2006; 45: 370–4
Molin L, Cutler TP, Helander I, et al. Comparative efficacy of calcipotriol (MC903) cream and betamethasone 17-valerate cream in the treatment of chronic plaque psoriasis: a randomized, double-blind, parallel group multicentre study. Calcipotriol Study Group. Br J Dermatol 1997; 136: 89–93
Bruce S, Epinette WW, Funicella T, et al. Comparative study of calcipotriene (MC 903) ointment and fluocinonide ointment in the treatment of psoriasis. J Am Acad Dermatol 1994; 31: 755–9
Cunliffe WJ, Berth-Jones J, Claudy A, et al. Comparative study of calcipotriol (MC 903) ointment and betamethasone 17-valerate ointment in patients with psoriasis vulgaris. J Am Acad Dermatol 1992; 26: 736–43
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol 2011 Jul; 65 (1): 137–74
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol 2009; 60: 643–59
Zeichner JA, Lebwohl MG, Menter A, et al. Optimizing topical therapies for treating psoriasis: a consensus conference. Cutis 2010; 86 (3 Suppl.): 5–31
Vectical® (calcitriol) ointment: US prescribing information. Fort Worth (TX): Galderma Laboratories L.P., 2009
Taclonex® (calcipotriene 0.005% and betamethasone dipropionate 0.064%) ointment: US prescribing information. Parsippany (NJ): LEO Pharma, Inc., 2010
Kragballe K, Austad J, Barnes L, et al. Efficacy results of a 52-week, randomised, double-blind, safety study of a calcipotriol/betamethasone dipropionate two compound product (Daivobet/Dovobet/Taclonex) in the treatment of psoriasis vulgaris. Dermatology 2006; 213: 319–26
Lebwohl M, Ortonne J-P, Andres P, et al. Calcitriol ointment 3 μg/g is safe and effective over 52 weeks for the treatment of mild to moderate plaque psoriasis. Cutis 2009; 83: 205–12
Highton A, Quell J. Calcipotriene ointment 0.005% for psoriasis: a safety and efficacy study. Calcipotriene Study Group. J Am Acad Dermatol 1995; 32: 67–72
Cullen SI. Long-term effectiveness and safety of topical calcipotriene for psoriasis: Calcipotriene Study Group. South Med J 1996; 89: 1053–6
Acknowledgments
Medical writing and editorial support were provided by Mark P. Bowes, PhD, medical writer, and Medisys Health Communications, and funded by Stiefel, a GSK company. These studies were sponsored by Stiefel, a GSK company.
Dr Feldman has received research, speaking and/or consulting support from GSK/Stiefel, Galderma Laboratories, Medicis, Abbott, Amgen, Astellas, Centocor, National Biological Corporation, Novartis, and Aventis. Dr Matheson received a grant from Stiefel, a GSK company, for participating in this clinical trial. Dr Bruce received a grant from Stiefel, a GSK company, as principal investigator in this clinical trial. Dr Grande served as a principal investigator at a study site and received compensation for this clinical trial, and participated in the investigator’s meeting for this trial. Dr Markowitz has no conflicts of interest to declare. Dr Kempers is an investigator for Stiefel, a GSK company, and was an investigator in this clinical trial. Thomas Brundage is an employee of Stiefel, a GSK company, and is a shareholder in GSK. Melody Wyres is an employee of Stiefel, a GSK company.
The authors gratefully acknowledge the U0267-301 Study Investigators: William Abramovits, Zoe Draelos, Francisco A. Kerdel, Alexa Kimball, Leon Kircik, Leonard J. Swinyer, Hector Wiltz; and the U0267-302 Study Investigators: Steven A. Davis, Sunil Dhawan, Ellen H. Frankel, David L. Kaplan, Phoebe Rich, Brett Shulman, James A. Solomon, Eduardo Tschen.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
An Erratum for this chapter can be found at http://dx.doi.org/10.2165/11630470-000000000-00000
An erratum to this article is available at http://dx.doi.org/10.2165/11630470-000000000-00000.
Rights and permissions
About this article
Cite this article
Feldman, S.R., Matheson, R., Bruce, S. et al. Efficacy and Safety of Calcipotriene 0.005% Foam for the Treatment of Plaque-Type Psoriasis. Am J Clin Dermatol 13, 261–271 (2012). https://doi.org/10.2165/11630710-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11630710-000000000-00000