Abstract
Background: Two Dutch observational studies (HARM[Hospital Admissions Related to Medication] and IPCI [Integrated Primary Care Information]) have shown that approximately 5% of all unplanned hospital admissions are associated with adverse drug events (ADEs), of which 40–46% are potentially preventable. These studies prompted the initiation of a Dutch multidisciplinary task force, which was assigned to reduce the number of prescriber-related hospital admissions related to medications (HARMs) in a quick-win way.
Objective: The aim of the study was to identify the most relevant ADEs and to develop a limited number of recommendations for concrete interventions, which should be feasible and relatively easy to convert into computerized drug safety alerts.
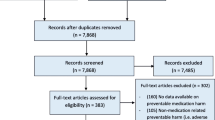
Method: To identify the major ADEs, crude data of HARM and IPCI were reanalysed and compared with different international studies, followed by structured literature searches for further characterization of the identified ADEs, their risk factors and potential risk-reduction strategies. Based on this information, the Task Force drew up general and drug-specific recommendations. As the recommendations of the Task Force are a mixture of evidenceand expert-based risk-reducing strategies, they have been graded in accordance with the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology.
Results: Seven pharmacologically predictable ADEs associated with ten drug classes were responsible for more than half of all potentially preventable hospital admissions in the IPCI and HARM studies, which was comparable to the results of international studies. Gastrointestinal and other bleedings were the most frequent ADE, followed by disturbances of diabetes mellitus control, electrolyte disturbances, fractures, renal insufficiency and heart failure. Nine general and 34 drug-specific recommendations were developed.
Conclusions: As HARMs constitute a significant public health problem, the Task Force underlines the need to implement its recommendations as soon as possible. They do not replace existing guidelines, but reinforce, complement and fine-tune existing Dutch and international guidelines. Further research is still required to assess the cost consequences and cost effectiveness of some recommendations, and to monitor the implementation of the recommendations and their effect on the incidence of potentially preventable HARMs.



Similar content being viewed by others
References
Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta analysis of observational studies. Pharm World Sci 2002 Apr; 24 (2): 46–54
Sturkenboom MCJM, Dieleman JP. Ziekenhuisopnames door bijwerkingen van geneesmiddelen-een inventarisatie. Eindrapport. Rotterdam: Erasmus MC, 2006
van derHooftCS, SturkenboomMC, vanGrootheestK, et al. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf 2006; 29 (2): 161–8
Hallas J, Harvald B, Gram LF, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 1990 Aug; 228 (2): 83–90
Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992 Jun; 27 (6): 538
Leendertse AJ, Egberts AC, Stoker LJ, et al. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med 2008 Sep 22; 168 (17): 1890–6
Van den Bemt PMLA, Egberts ACG, Leendertse A. Hospital Admissions Related to Medication (HARM). Een prospectief, multicenter onderzoek naar geneesmiddel gerelateerde ziekenhuisopnames. Eindrapport. Utrecht: Division of Pharmacoepidemiology & Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences, 2006
Kramer MS, Leventhal JM, Hutchinson TA, et al. An algorithm for the operational assessment of adverse drug reactions: I. Background, description, and instructions for use. JAMA 1979 Aug 17; 242 (7): 623–32
De Smet PAGM, Denneboom W, Kramers C, et al. A composite screening tool for medication reviews of outpatients: general issues with concrete examples. Drugs Aging 2007; 24 (9): 733–60
Stricker BH, Psaty BM. Detection, verification, and quantification of adverse drug reactions. BMJ 2004 Jul 3; 329 (7456): 44–7
Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet 2004 May 22; 363 (9422): 1728–31
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008 Apr 26; 336 (7650): 924–6
GRADE working group. The Grading of Recommendations Assessment, Development and Evaluation (short GRADE) Working Group [online]. Available from URL: http://www.gradeworkinggroup.org [Accessed 2010 Aug 16]
Guyatt GH, Cook DJ, Jaeschke R, et al. Grades of recommendation for antithrombotic agents: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed.). Chest 2008 Jun; 133 Suppl. 6: 123–31S
Warle′-Van Herwaarden MF, Kramers C, Sturkenboom MC, et al. Targeting outpatient drug safety: recommendations of the Dutch HARM-Wrestling Task Force. Den Haag: Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie, 2010 [online]. Available from URL: http://www.knmp.nl/downloads/medicijnenzorgverlening/medicatieveiligheid/harmwrestlingEnglishcopyrightKNMP.pdf/view [Accessed 2012 Jan 3]
Visser LE, Graatsma HH, Stricker BH. Contraindicated NSAIDs are frequently prescribed to elderly patients with peptic ulcer disease. Br J Clin Pharmacol 2002 Feb; 53 (2): 183–8
van der Linden CM, Kerskes MC, Bijl AM, et al. Represcription after adverse drug reaction in the elderly: a descriptive study. Arch Intern Med 2006 Aug 14; 166 (15): 1666–7
Zhang M, Holman CD, Preen DB, et al. Repeat adverse drug reactions causing hospitalization in older Australians: a population-based longitudinal study 1980-2003. Br J Clin Pharmacol 2007 Feb; 63 (2): 163–70
Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998 Aug; 105 (2): 91–9
Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004 Sep; 126 Suppl. 3: 204–33S
Feenstra J, Heerdink ER, Grobbee DE, et al. Association of nonsteroidal anti-inflammatory drugs with first occurrence of heart failure and with relapsing heart failure: the Rotterdam Study. Arch InternMed 2002 Feb 11; 162 (3): 265-70
Centraal Begeleidings Orgaan (CBO). Richtlijn: NSAIDgebruik en preventie van maagschade. 2003 [online]. Available from URL: http://www.cbo.nl/Downloads/259/richtlijnnsaid.pdf [Accessed 2012 Jan 3]
Raynor DK, Blenkinsopp A, Knapp P, et al. A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines. Health Technol Assess 2007 Feb; 11 (5): iii, 1–160
Anonymous. HARM-WRESTLING: een voorstel van de Expertgroep Medicatieveiligheid m.b.t. concrete interventies die de extramurale medicatieveiligheid op korte termijn kunnen verbeteren. Den Haag: Ministerie van Volksgezondheid, Welzijn en Sport, 2009
Schneeweiss S,Hasford J, GottlerM, et al. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol 2002 Jul; 58 (4): 285–91
Howard RL, Avery AJ, Slavenburg S, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007; 63: 136–47
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004 Jul 3; 329 (7456): 15–9
Buurma H, De Smet PAGM, Kruijtbosch M, et al. Disease and intolerability documentation in electronic patient records. Ann Pharmacother 2005 Oct; 39 (10): 1640–6
Buurma H, Schalekamp T, Egberts ACG, et al. Compliance with national guidelines for the management of drug-drug interactions in Dutch community pharmacies. Ann Pharmacother 2007; 41 (12): 2024–31
Ashcroft DM, Parker D. Development of the Pharmacy Safety Climate Questionnaire: a principal components analysis. Qual Saf Health Care 2009 Feb; 18 (1): 28–31
Ashcroft DM, Morecroft C, Parker D, et al. Safety culture assessment in community pharmacy: development, face validity, and feasibility of the Manchester Patient Safety Assessment Framework. Qual Saf Health Care 2005 Dec; 14 (6): 417–21
De Smet PAGM, Dautzenberg M. Repeat prescribing: scale, problems and quality management in ambulatory care patients. Drugs 2004; 64: 1779–800
Kuijpers MA, van Marum RJ, Egberts AC, et al. Relationship between polypharmacy and underprescribing. Br J Clin Pharmacol 2008 Jan; 65 (1): 130–3
Denneboom W, Dautzenberg M, Grol RP, et al. Analysis of polypharmacy in older patients in primary care using a multidisciplinary expert panel. Br J Gen Pract 2006; 56: 504–10
Cote GA, Rice JP, Bulsiewicz W, et al. Use of physician education and computer alert to improve targeted use of gastroprotection among NSAID users. Am J Gastroenterol 2008 May; 103 (5): 1097–103
Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 2004 Aug 5; 351 (6): 585–92
Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors. How much should we worry? Arch Intern Med 1998 Jan 12; 158 (1): 26–32
Verhave JC, Kramers C, Wetzels JFM. Nauwkeurige indruk van de nierfunctie. Nieuwe formule leidt tot betere schattingen. Pharm Weekbl 2007; 142 (40): 18–21, 56
Centraal Begeleidings Orgaan (CBO). Osteoporose Tweede herziene richtlijn. Utrecht: Kwaliteitsinstituut voor de Gezondheidszorg CBO, 2002
De Smet PA. Hospital admissions related to medications and implementing guidelines. Arch InternMed 2009 Apr 27; 169 (8): 810–1
Fugh-Berman A, Ahari S. Following the script: how drug reps make friends and influence doctors. PLoS Med 2007 Apr; 4 (4): e150
Acknowledgements
This study was prepared on behalf of the HARM-Wrestling Task Force: P.A.G.M De Smet, Pharmacist/Clinical Pharmacologist, PharmD, PhD (Chair); P.M.L.A. van den Bemt, PhD, Hospital Pharmacist (member of the HARM study group and the Dutch Association of Hospital Pharmacists); H. Buurma, PhD, Community Pharmacist (member of the Royal Dutch Association for the Advancement of Pharmacy); F. Boersma, PhD, Nursing Home Physician (member of the Dutch Association for Nursing Home Physicians); H. Folmer, MSc, General Practitioner (member of the Dutch National Society of General Practitioners); J.L.M. van Klundert,MSc,MD (Senior Advisor of the Dutch Order of Medical Specialists); M.E.A.P. Kokenberg, MSc, Pharmacist (Secretary of the preparatory group); R.J. van Marum, PhD, Clinical Geriatrician/Clinical Pharmacologist (member of the Dutch Geriatrics Society); M. Sturkenboom, PharmD, PhD, Pharmacist/Clinical Epidemiologist (member of the IPCI study group).
On behalf of all authors, the corresponding author (Peter A.G.M. De Smet) gives permission to publish this article in Drug Safety and to exploit all subsidiary rights.
All authors declare that there were no competing interests.
Peter De Smet contributed to conception and study design. Patricia van den Bemt and Miriam Sturkenboom provided access to the anonymized crude data of the HARM and IPCI studies. Margaretha Warle′-van Herwaarden and Peter De Smet combined these into a single database and used it for further analysis. Peter De Smet collected and aggregated the background literature. The Task Force (including Patricia van den Bemt, Miriam Sturkenboom, Margaretha Warle′-van Herwaarden, Cees Kramers and Peter De Smet) interpreted the data and developed the recommendations. All authors contributed to the drafting of the manuscript and approved the final version of the report.
This multidisciplinary consensus was funded by the Dutch Ministry of Health, Welfare and Sport. This played no role in the design and conduction of the study, or in the collection, management, analysis and interpretation of the data, or review or approval of the manuscript.
We would like to thank the other members of the Task Force (H. Buurma, F. Boersma, H. Folmer, J.L.M. van Klundert, M.E.A.P. Kokenberg and R.J. van Marum). Furthermore, we would like to thank A.M. Linssen for her assistance in creating a schematic overview of the guidelines for secondary prevention of arterial thrombosis, and M. Roukens for her correction of typographic errors on the final report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herwaarden, M.F.Wv., Kramers, C., Sturkenboom, M.C. et al. Targeting Outpatient Drug Safety. Drug Saf 35, 245–259 (2012). https://doi.org/10.2165/11596000-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11596000-000000000-00000