Abstract
Background: Two phase II trials (POWER 1 and 2) have demonstrated that darunavir co-administered with low-dose ritonavir (DRV/r) provides significant clinical benefit compared with control protease inhibitors (PIs) in highly treatment-experienced, HIV-1-infected adults, when co-administered with optimized background therapy (OBR).
Objective: To determine whether DRV/r is cost effective compared with control PIs, from the perspective of Belgian, Italian, Swedish and UK reimbursement authorities, when used in treatment-experienced patients similar to those included in the POWER 1 and 2 trials.
Methods: An existing Markov model containing health states defined by CD4 cell count ranges (>500, 351–500, 201–350, 101–200, 51–100 and 0–50 cells/mm3) and death was adapted for use in four European healthcare settings. Baseline demographics, CD4 cell count distribution and antiretroviral drug usage reflected those reported in the POWER 1 and 2 trials. Virological/immunological response rates and matching transition probabilities over the patients lifetime were based on results from the POWER trials and published data. After treatment failure, patients were assumed to switch to a tipranavir-containing regimen plus OBR. For each CD4 cell count range, utility values and HIV-related mortality rates were obtained from the published literature. National all-cause mortality data and published data on the increased risk of non HIV-related mortality in HIV-infected individuals were taken into account in the model. Data from observational studies conducted in each healthcare setting were used to determine resource-use patterns and costs associated with each CD4 cell count range. Unit costs were derived from official local sources; a lifetime horizon was taken and discount rates were selected based on local guidelines.
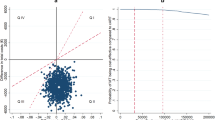
Results: In the base-case analysis, quality-adjusted life-year (QALY) gains of up to 1.397 in Belgium, over 1.171 in Italy, 1.142 in Sweden and 1.091 in the UK were predicted when DRV/r-based therapy was used instead of control PI-based treatment. The base-case analyses predicted an incremental costeffectiveness ratio (ICER) of h11 438/QALY in Belgium, h12 122/QALY in Italy, h10 942/QALY in Sweden and h16 438/QALY in the UK. Assuming an acceptability threshold of h30 000/QALY, DRV/r-based therapy remained cost effective over all parameter ranges tested in extensive one-way sensitivity analyses. Probabilistic sensitivity analysis revealed a 95% (Belgium), 97% (Italy), 92% (Sweden) or 78% (UK) probability of attaining an ICER below this threshold.
Conclusion: From four European payer perspectives, DRV/r-based antiretroviral therapy is predicted to be cost effective compared with currently available control PIs, when both are used with an OBR in treatment-experienced, HIV-1-infected adults who failed to respond to more than one PI-containing regimen.














Similar content being viewed by others
References
Palella Jr FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338: 853–60
Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the Euro SIDA study: an observational study. Lancet 2003; 362: 22–9
Hamers FF, Downs AA. The changing face of the HIV epidemic in western Europe: what are the implications for public health policies? Lancet 2004; 364: 83–94
Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis 2006; 194: 11–19
Pomerantz RJ, Horn DL. Twenty years of therapy for HIV-1 infection. Nat Med 2003; 9: 867–73
Yazdanpanah Y, Goldie SJ, Losina E, et al. Lifetime cost of HIV care in France during the era of highly active antiretroviral therapy. Antiviral Ther 2002; 7: 257–66
Levy AR, James D, Johnston KM, et al. The direct costs of HIV/AIDS care. Lancet Infect Dis 2006; 6: 171–7
Beck EJ, Mandalia S. The cost of HIV treatment and care in England since HAART — part 1. Br J Sex Med 2006; 27: 19–23
Mocroft A, Ledergerber B, Viard JP, et al. Time to virological failure of 3 classes of antiretrovirals after initiation of highly active antiretroviral therapy: results from the EuroSIDA study group. J Infect Dis 2004; 190: 1947–56
Sabin CA, Hill T, Lampe F, et al. Treatment exhaustion of highly active antiretroviral therapy (HAART) among individuals infected with HIV in the United Kingdom: multicentre cohort study. BMJ 2005; 330: 695
Gazzard B, Bernard AJ, Boffito M, et al. British HIV Association (BHIVA) guidelines for the treatment of HIV infected adults with antiretroviral therapy (2006). HIV Med 2006; 7: 487–503
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2009 Dec 1 [online]. Available from URL: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Accessed 2009 Dec 10]
Hammer SM, Saag MS, Schechter M, et al. Treatment for Adult HIV Infection: 2006 recommendations of the International AIDS Society — USA Panel. JAMA 2006; 296: 827–43
Deeks SG, Barbour JD, Grant RM, et al. Duration and predictors of CD4 T-cell gains in patients who continue combination therapy despite detectable plasma viremia. AIDS 2002; 16: 201–7
Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 2004; 364: 51–62
Gazzard B, BHIVA Writing Committee. British HIV Association (BHIVA) guidelines for the treatment of HIV infected adults with antiretroviral therapy (2005). HIV Med 2005 Jul; 6 Suppl. 2: 1–61
Koh Y, Nakata H, Maeda K, et al. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob Agents Chemother 2003; 47: 3123–9
De Meyer S, Azijn H, Surleraux D, et al. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother 2005; 49: 2314–21
Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavirritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 2007; 369: 1169–78
U.S. Food and Drug Administration (FDA). Press announcements: FDA approves new HIV treatment for patients who do not respond to existing drugs; 2006. Jun 23 [online]. Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108676.htm [Accessed 2009 Dec 10]
European Medicines Agency (EMEA). Press release: meeting highlights from the Committee for Medicinal Products for Human Use; 2006 Dec 1114 [online]. Available from URL: (http://www.emea.europa.eu/pdfs/human/press/pr/50697706en.pdf) [Accessed 2009 Dec 10]
European Commission. Community register of medicinal products for human use: Prezista; [online]. Available from URL: http://ec.europa.eu/enterprise/pharmaceuticals/register/h380.htm [Accessed 2009 Dec 10]
Mauskopf J, Brogan A, Smets E, et al. Cost-effectiveness of darunavir (TMC114)/ritonavir compared with currently available protease inhibitors in treatment-experienced HIV patients [abstract P51]. 8th International Congress on Drug Therapy in HIV Infection; 2006 Nov 1216; Glasgow, UK
D’Aquila RT, Schapiro JM, Brun-Vezinet F, et al. Drug resistance mutations in HIV-1. Top HIV Med 2003; 11: 92–6
Tibotec BVBA. Darunavir: 2.5 clinical overview. Document 39881. Tibotec Therapeutics, Bridgewater, NJ, USA. Confidential information, 2005 Dec 7
Euroguidelines Group. Summary European guidelines for the clinical management and treatment of HIV infected adults in Europe [summary, pocket version]. 10th European AIDS Conference (EACS) Symposium; 2005 Nov 1017; Dublin, Ireland
Phillips AN, Lundgren JD. The CD4 lymphocyte count and risk of clinical progression. Curr Opin HIV AIDS 2006; 1: 43–9
Phillips AN, Dunn D, Sabin C, et al. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS 2005; 19: 487–94
Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microl in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis 2005; 41: 361–72
Hicks C, Cahn P, Cooper DA, et al. Durable efficacy of tipranavirritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multidrug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised openlabel trials. Lancet 2006; 368: 466–75
Tarwater PM, Margolick JB, Jin J, et al. Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr 2001; 27: 168–75
Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 Tlymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med 2003; 163: 2187–95
Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS 2003; 17: 1907–15
Smith CJ, Sabin CA, Lampe FC, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS 2003; 17: 963–9
Garcia F, de Lazzari E, Plana M, et al. Long-term CD4+ T-cell response to highly active antiretroviral therapy according to baseline CD4+ T-cell count. J Acquir Immune Defic Syndr 2004; 36: 702–13
Jensen-Fangel S, Pedersen L, Pedersen C, et al. Low mortality in HIV-infected patients starting highly active antiretroviral therapy: a comparison with the general population. AIDS 2004; 18: 89–97
Mauskopf J, Brogan A, Martin S, et al. Cost-effectiveness of darunavir/ritonavir in highly treatment-experienced, HIV-1-infected adults in the USA. PharmacoEconomics 2010; 28 Suppl. 1: 83–105
Tibotec Pharmaceuticals. Data on file, 2006
Cahn P,RESIST 2 Study Team. 24-week data fromRESIST 2: Phase 3 study of the efficacy and safety of background therapy plus tipranavir/ritonavir (TPV/r) or optimized ritonavir-boosted standard-of-care (SOC) comparator PI (CPI) in a large randomized multicenter trial in treatmentexperienced HIV+ patients [abstract PL14.3]. 7th International Congress on Drug Therapy in HIV Infection; 2004 Nov 1418; Glasgow, UK
Pozniak A, Jayaweera D, Hoy J, et al. Efficacy of darunavir/ritonavir in treatment-experienced HIV-1-infected patients at 96 weeks in the POWER 1 and 2 trials [abstract P7.2/07]. 11th European AIDS Conference; 2007 Oct 2427; Madrid, Spain
Hicks C, RESIST 1 Study Team. A Phase 3 randomized, controlled, open-label multicenter trial comparing tipranavir/ritonavir (TPV/r) to an optimized comparator protease inhibitor/r (CPI/r) regimen in antiretroviral (ARV) experienced patients: 24-week data [abstract 3726]. 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2004 Oct 31Nov 2; Washington, DC, USA
Hicks C, RESIST 1 Study Team. Phase 3 comparison of TPV/r and standard-of-care boosted-comparator PI (CPI/r) at 24 weeks [abstract H-1137a]. 46th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2006 Sep 2730; San Francisco, CA, USA
World Health Organization. Mortality database: tables. Italy; 2004 [online]. Available from URL: http://www.who.int/healthinfo/morttables/en/index.html [Accessed 2009 Dec 10]
Statistics Sweden. Tables on the population in Sweden 2005. Official Statistics of Sweden; 2006 [online]. Available from URL: http://www.scb.se/statistik/_publikationer/BE0101_2005A01_BR_BE0106TAB.pdf [Accessed 2009 Dec 10]
Belgian National Institute of Statistics (NIS). Démographie mathématique: tables de mortalité; 2001 [online]. Available from URL: http://statbel.fgov.be/fr/binaries/p238y2001_fr_tcm326-39345.pdf [Accessed 2009 Dec 10]
UK Government Actuary’s Department. Life tables; 2005 [online]. Available from URL: http://www.gad.gov.uk/Demography%20Data/Life%20Tables/index.html [Accessed 2009 Dec 10]
Simpson KN, Luo MP, Chumney E, et al. Cost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infection. HIV Clin Trials 2004; 5: 294–304
Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–108
Hill AM, Clotet B, Johnson M, et al. Costs to achieve undetectable HIV RNA with darunavir-containing highly active antiretroviral therapy in highly pretreated patients: the POWER experience. PharmacoEconomics 2010; 28 Suppl. 1: 69–81
Caekelbergh K, Moeremans K, Annemans L, et al. Cost of care for HIV/AIDS in Belgium according to disease stage [abstract P19.5/01]. 11th European AIDS Conference; 2007 Oct 2427; Madrid, Spain
Tramarin A, Campostrini S, Postma MJ, et al. Amulticentre study of patient survival, disability, quality of life and cost of care: among patients with AIDS in northern Italy. PharmacoEconomics 2004; 22: 43–53
Istituto Nazionale di Statistica. Indices of Consumer Prices; [online]. Available from URL: http://www.istat.it [Accessed 2009 Dec 10]
Ghatnekar O, Gisslén M, Hjortsberg C, et al. Medical resource use and cost of HIV-related care in the HAART-era at a university clinic in Sweden [abstract P19.10/01]. 11th European AIDS Conference; 2007 Oct 2427; Madrid, Spain
Petrou S, Dooley M, Whitaker L, et al. The economic cost of caring for people with HIV infection and AIDS in England and Wales. PharmacoEconomics 1996; 9: 332–40
Personal Social Services Research Unit (PSSRU). Unit costs of health and social care 2008. Inflation indices; [online]. Available from URL: http://www.pssru.ac.uk/uc/uc2008contents.htm [Accessed 2009 Dec 10]
Statistics Belgium. Directorate-general statistics and economic information; [online]. Available from URL: http://statbel.fgov.be [Accessed 2009 Dec 10]
Statistics Sweden. Consumer price index: tables and charts; [online]. Available from URL: http://www.scb.se/Pages/TableAndChart____33907.aspx [Accessed 2009 Dec 10]
Capri S, Ceci A, Terranova L, et al. Guidelines for economic evaluations in Italy: recommendations from the Italian group of pharmacoeconomic studies. Drug Information J 2001; 35: 189–201
Tandvårds-och lä kemedelsförmånsverket (TLV). General guidelines for economic evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2). 2003 Apr 24 [online]. Available from URL: http://www.tlv.se/Upload/English/ENG-lfnar-2003-2.pdf [Accessed 2009 Dec 10]
Cleemput I, Crott R, Vrijens F, et al. Voorlopige richtlijnen voor farmaco-economisch onderzoek in België. Brussel: Federaal Kenniscentrum voor de gezondheidszorg (KCE); KCE Reports vol. 28A. Ref. D/2006/10.273/10; 2006 [online]. Available from URL: http://www.kce.fgov.be/Download.aspx?ID=494 [Accessed 2009 Dec 10]
Cook J, Dasbach E, Coplan P, et al. Modeling the long-term outcomes and costs of HIV antiretroviral therapy using HIV RNA levels: application to a clinical trial. AIDS Res Hum Retroviruses 1999; 15: 499–508
Schackman BR, Goldie SJ, Freedberg KA, et al. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making 2002; 22: 27–38
King Jr JT, Justice AC, Roberts MS, et al. Long-term HIV/AIDS survival estimation in the highly active antiretroviral therapy era. Med Decis Making 2003; 23: 9–20
World Health Organization (WHO). Table: Threshold values for intervention cost-effectiveness by Region; [online]. Available from URL: http://www.who.int/choice/costs/CER_levels/en/index.html [Accessed 2009 Dec 10]
Towse A. What is NICE’s threshold? An external view. Chapter 2. In: Devlin N, Towse A, editors. Cost effectiveness thresholds: economic and ethical issues. London: King’s Fund/Office for Health Economics, 2002
Birch S, Gafni A. On being NICE in the UK: guidelines for technology appraisal for the NHS in England and Wales. Health Econ 2002; 11: 185–91
Birch S, Gafni A. The ‘NICE’ approach to technology assessment: an economics perspective. Health Care Manag Sci 2004; 7: 35–41
Gafni A, Birch S, NICE; National Health Service. NICE methodological guidelines and decision making in the National Health Service in England and Wales. PharmacoEconomics 2003; 21: 149–57
Stoll M, Schulte E, Claes C, et al. The cost of an HIV patient. Lack of funds for optimal treatment? [Article in German]. MMW Fortschr Med 2001; 143 Suppl. 1: 72–7
Garattini L, Tediosi F, Di Cintio E, et al. Resource utilization and hospital cost ofHIV/AIDS care in Italy in the era of highly active antiretroviral therapy. AIDS Care 2001; 13: 733–41
Bozzette SA, Joyce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med 2001; 344: 817–23
Mouton Y, Alfandari S, Valette M, et al. Impact of protease inhibitors on AIDS-defining events and hospitalizations in 10 French AIDS reference centres. Federation National des Centres de Lutte contre le SIDA. AIDS 1997; 11: F101–5
Hornberger J, Green J, Wintfeld N, et al. Cost-effectiveness of enfuvirtide for treatment-experienced patients with HIV in Italy. HIV Clin Trials 2005; 6: 92–102
European Medicines Evaluation Agency (EMEA) Committee for Medicinal Products for Human Use (CHMP). European Public Assessment Report (EPAR): Aptivus. EMEA/H/C/631; 2007 [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/ap tivus/32004705en1.pdf [Accessed 2009 Dec 10]
Hill A, Moyle G. Relative antiviral efficacy of ritonavir boosted darunavir and ritonavir-boosted tipranavir vs. control protease inhibitor in the POWER and RESIST trials. HIV Med 2007; 8: 259–64
Gathe J, DeJesus E, Falcon R, et al. Examination of factors influencing response to darunavir combined with low-dose ritonavir in POWER 1, 2, and 3: pooled 48-week analysis [abstract 66]. Frontiers in Drug Development for Antiretroviral Therapies; 2006 Dec 10–14; Cancun, Mexico
Decock RC, Depoorter AM, De Graeve D, et al. Direct costs of health care for HIV/AIDS patients in Belgium. AIDS Care 2001; 13: 721–31
Stoll M, Claes C, Schulte E, et al. Direct costs for the treatment of HIV-infection in a German cohort after the introduction of HAART. Eur J Med Res 2002; 7: 463–71
Levy A, Annemans L, Tramarin A, et al. The impact of disease stage on direct medical costs of HIV infection: a review of the international literature. PharmacoEconomics 2010; 28 Suppl. 1: 35–47
80. Lalezari J, Goodricj J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [abstract 104bLB]. 14th Conference on Retroviruses and Opportunistic Infections; 2007 Feb 25–28; Los Angeles, CA, USA
Madruga JV, Cahn P, Grinsztejn B, on behalf of the DUET-1 study group. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results froma randomised, double-blind, placebo-controlled trial. Lancet 2007; 370: 29–38
Lazzarin A, Campbell T, Clotet B. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007; 370: 39–48
Kahn JO, Lagakos SW, Richman DD. A controlled trial comparing continued zidovudine with didanosine in human immunodeficiency virus infection. The NIAID AIDS Clinical Trials Group. N Engl J Med 1992; 327: 581–7
Randomised trial of addition of lamivudine or lamivudine plus loviride to zidovudine-containing regimens for patients with HIV-1 infection: the CAESAR trial. Lancet 1997; 349: 1413–21
Delta: a randomised double-blind controlled trial comparing combinations of zidovudine plus didanosine or zalcitabine with zidovudine alone in HIV-infected individuals. Lancet 1996; 348: 283–91
Lacey L, Youle M, Trueman P, et al. A prospective evaluation of the cost effectiveness of adding lamivudine to zidovudine-containing antiretroviral treatment regimens in HIV infection. European perspective. PharmacoEconomics 1999; 15 Suppl. 1: 39–53
Food and Drug Administration (FDA), Division of Antiviral Drug Products. Guidance for industry: antiretroviral drugs using plasma HIV RNA measurements. Clinical considerations for accelerated and traditional approval, prepared by the: Office of Drug Evaluation IV in the Centre for Drug Evaluation and Research (CDER), Appendix B; 2002 Oct
European Medicines Evaluation Agency (EMEA) Committee for Medicinal Products for Human Use (CHMP). Draft: guideline on the clinical development of medicinal products for the treatment of HIV infection. London, 18 October 2007. EMEA/CPMP/EWP/633/02 Rev. 2; 2007 Oct 18 [online]. Available from URL: http://www.emea.europa.eu/pdfs/human/ewp/063302enrev2.pdf [Accessed 2009 Dec 10]
Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIVinfected patients in TITAN: a randomised controlled phase III trial. Lancet 2007; 370: 49–58
Berger DS, Northland R, Scribner A, et al. Effect of baseline factors on virological response to darunavir/r and lopinavir/r at week 48 in TITAN [abstract P7.3/27]. 11th European AIDS Conference; 2007 Oct 24–27; Madrid, Spain
Ortiz R, Dejesus E, Khanlou H, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS 2008; 22: 1389–97
Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr 2002 Dec 15; 31 Suppl. 3: S123–7
Maggiolo F, Ripamonti D, Gregis G, et al. Once-a-day therapy for HIV infection: a controlled, randomized study in antiretroviral-naive, HIV-1-infected patients. Antivir Ther 2003; 8: 339–46
Negredo E, Bonjoch A, Clotet B. Benefits and concerns of simplification strategies in HIV-infected patients. J Antimicrob Chemother 2006; 58: 235–42
Acknowledgements
The authors wish to thank the investigators and the patients and their families for their participation and support during the POWER studies. Special thanks go to Josephine Mauskopf and Anita Brogan from the Research Triangle Institute (RTI), who developed the model structure and kindly provided technical support. The authors are also grateful to the investigators who participated in the Belgian cost-of-illness study (Robert Colebunders, Institute for Tropical Medicine, Antwerp, Belgium; Nathan Clumeck, Saint-Pierre University Hospital, Brussels, Belgium; Eric Van Wijngaerden, University Hospital Leuven, Belgium; Bernard Vandercam, Saint-Luc University Hospital, Brussels, Belgium; Michel Moutschen, Sart Tilman University Hospital, Liège, Belgium) and to the team led by Magnus Gisslén at the Department of Infectious Diseases within the Sahlgrenska Academy at Göteborg University, Gothenburg, Sweden, who participated in the Swedish cost-of-illness study. The authors also specially thank Tony Vangeneugden and Ben Van Baelen for analysing and providing the POWER trial data in line with the model structure and required inputs, and Eric Lefebvre, Martine De Pauw, Frederic Godderis, Piet De Doncker and the rest of the darunavir study team for their contributions. They would like to acknowledge Catherine McCarthy Bragg (medical writer, Gardiner-Caldwell Communications, Macclesfield, UK), for her assistance in editing the manuscript and collating author contributions. This project was financially supported by Johnson & Johnson Pharmaceutical Services, Janssen Cilag SpA (Italy), Janssen-Cilag NV (Belgium) and Janssen-Cilag AB (Sweden).
KM is an employee of IMS Health. IMS Health received an unrestricted grant from Johnson & Johnson for conducting this research. LA has received consultancy fees from Johnson & Johnson. ML is an employee of Janssen-Cilag AB, and owns stock options in Johnson & Johnson. GA is an employee of Janssen-Cilag SpA. VW is an employee of Janssen-Cilag NV. LH is an employee of Tibotec. ES is an employee of Johnson & Johnson Pharmaceutical Services, Beerse, Belgium, and owns stock options and shares in this company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moeremans, K., Annemans, L., Löthgren, M. et al. Cost Effectiveness of Darunavir/Ritonavir 600/100mg bid in Protease Inhibitor-Experienced, HIV-1-Infected Adults in Belgium, Italy, Sweden and the UK. Pharmacoeconomics 28 (Suppl 1), 107–128 (2010). https://doi.org/10.2165/11587480-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11587480-000000000-00000