Abstract
Background
Several treatment options are available for patients with type 2 diabetes mellitus who are making the transition from oral antidiabetes drugs (OADs) to insulin. Two options currently recommended by the Canadian Diabetes Association for initiating insulin therapy in patients with type 2 diabetes who are no longer responsive to OADs alone are insulin glargine plus OADs, and premixed insulin therapy only. Because of differences in efficacy, adverse events (such as hypoglycaemia) and acquisition costs, these two treatment options may lead to different long-term clinical and economic outcomes.
Objective
To determine the cost effectiveness of insulin glargine plus OADs compared with premixed insulin without OADs in insulin-naive patients with type 2 diabetes in Canada.
Methods
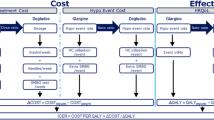
Using treatment effects taken from a published clinical trial, the validated IMS-CORE Diabetes Model was used to simulate the long-term cost effectiveness of insulin glargine with OADs, versus premixed insulin. Input treatment effects for the two therapeutic approaches were based on changes in glycosylated haemoglobin A1c (HbA1c) at clinical trial endpoint, and hypoglycaemia rates. The analysis was conducted from the perspective of the Canadian Provincial payer. Direct treatment and complication costs were obtained from published sources (primarily from Ontario) and reported in $Can, year 2008 values. All base-case costs and outcomes were discounted at 5% per year. Sensitivity analyses were conducted around key parameters and assumptions used in the study. Outcomes included direct medical costs associated with both treatment and diabetes-related complications. Cost-effectiveness outcomes included total average lifetime (35 years) costs, life expectancy (LE), QALYs and incremental cost-effectiveness ratios (ICERs).
Results
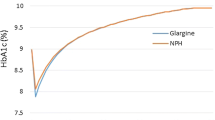
Base-case analyses showed that, compared with premixed insulin only, insulin glargine in combination with OADs was associated with a 0.051-year increase in LE and a 0.043 increase in QALYs. Insulin glargine plus OADs showed a very slight increase in total direct costs ($Can343 ± 2572), resulting in ICERs of $Can6750 per life-year gained (LYG) and $Can7923 per QALY gained. However, considerable uncertainty around the ICERs was demonstrated by insulin glargine having a 50% probability of being cost effective at a willingness-to-pay threshold of $Can10 000 per QALY, and a 54% probability at a $Can20 000 threshold. Base-case results were most sensitive to assumed disutilities for hypoglycaemic events, to the assumed effect of insulin glargine plus OADs on HbA1c, and to its assumed acquisition costs.
Conclusions
These findings should be interpreted within the context of a large degree of uncertainty and of several study limitations that include a single clinical trial as the source for primary treatment assumptions and a single province as the source for most cost inputs. Under current study assumptions and limitations, insulin glargine plus OADs was projected to be a cost-effective option, compared with premixed insulin only, for the treatment of insulin-naive patients with type 2 diabetes unresponsive to OADs. Additional work is needed to examine the generalizability of the findings to individual jurisdictions of the Canadian healthcare system.






Similar content being viewed by others
References
Canadian Diabetes Association. The prevalence and cost of diabetes [online]. Available from URL: http://www.diabetes.ca/about-diabetes/what/prevalence/ [Accessed 2009 Dec 1]
Public Health Agency of Canada. Diabetes [online]. Available from URL: http://www.phac-aspc.gc.ca/ccdpc-cpcmc/diabetes-diabete/english/pubs/ndfs-fnrd07-eng.html [Accessed 2008 Jun 1]
Canadian Optimal Medication Prescribing and Utilization Services. An economic evaluation of insulin analogues for the treatment of patients with type 1 and type 2 diabetes mellitus in Canada. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health (CADTH), 2008 [online]. Available from URL: http://www.cadth.ca/index.php/en/compus/insulin-analogs/reports [Accessed 2009 Sep]
Canadian Optimal Medication Prescribing and Utilization Services. Cost-effectiveness of blood glucose test strips in the management of adult patients with diabetes mellitus. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health (CADTH), 2009 [online]. Available from URL: http://www.cadth.ca/index.php/en/compus/insulin-analogs/reports [Accessed 2009 Sep]
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complication in patients with type 2 diabetes (UKPDS 33) [published erratum appears in Lancet 1999; 354 (9178): 602]. Lancet 1998; 352(9131): 837–53
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008; 32(1 Suppl.): 1–201S
Henderson JN, Allen KV, Deary IJ, et al. Hypoglycaemia in insulin-treated type 2 diabetes: frequency, symptoms and impaired awareness. Diabet Med 2003; 20(12): 1016–21
Janka HU, Plewe G, Riddle MC, et al. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care 2005; 28: 254–9
Palmer AJ, Roze S, Valentine WJ, et al. The CORE diabetes model: projecting long term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision making. Curr Med Res Opin 2004; 20(1 Suppl.): 5–26S
Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004; 20(1 Suppl.): 27S–40S
D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J 2000; 139(2 Pt 1): 272–81
American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care 2004; 27(9): 2262–5
Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES 1999–2000 dataset: diabetes survey. Hyattsville (MD): CDC, 2002 [online]. Available from URL: http://www.cdc.gov/nchs/data/nhanes/spq-di.pdf [Accessed 2009 Jan 1]
O’Reilly D, Hopkins R, Blackhouse G, et al. Development of an Ontario Diabetes Economic Model (ODEM) and application to a multidisciplinary primary care diabetes management program. Hamilton (ON): Program for Assessment of Technology in Health (PATH), 2006 [online]. Available from URL: http://www.path-hta.ca/diabetes.pdf [Accessed 2009 Jan 1]
Statistics Canada. Visible minority population, by age group. In: 2001 census of Canada. Ottawa (ON): Statistics Canada, 2007
Harris MI, Cowie CC, Stern MP. Diabetes in America. 2nd ed. [NIH publication no. 95-1468]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases, 1995 [online]. Available from URL: http://diabetes.niddk.nih.gov/dm/pubs/america/contents.htm [Accessed 2009 Feb 1]
Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care 2000; 23(6): 754–8
Moss SE, Klein R, Klein BE. The prevalence and incidence of lower extremity amputation in a diabetic population. Arch Inter Med 1992; 152(3): 610–6
Brändle M, Zhou H, Smith BR et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003; 26(8): 2300–4
Turner RC, Cull CA, Frighi V, UKPDS Study Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999; 281(21): 2005–12
Statistics Canada. Life tables, Canada, provinces and territories: 2000–2002. Ottawa (ON): Statistics Canada, 2006 [online; subscriber only]. Available from URL: http://dsp-psd.pwgsc.gc.ca/Collection/Statcan [Accessed 2009 Dec 1]
Royal Bank of Canada. Inflation calculator [online]. Available from URL: http://www.bankofcanada.ca/en/rates/inflation_calc.html [Accessed 2009 Jun 1]
Sullivan PW, Ghush V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006; 26(4): 410–20
Alberta Health and Wellness. Health costing in Alberta: 2006 annual report. Edmonton (AB): Alberta Health and Wellness, 2008
Caro J, Migliaccio-Walle K, Ishak KJ, et al. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database [abstract]. BMC Cardiovas Disord 2005; 5: 14
Hart HE, Redekop WK, Berg M, et al. Factors that predicted change in health-related quality of life were identified in a cohort of diabetes mellitus type 1 patients. J Clin Epidemiol 2005; 58(11): 1158–64
Manns B, Johnson JA, Taub K, et al. Quality of life in patients with end-stage renal disease over time: the impact of dialysis modality and other important determinants [working paper report no. 02-05]. Edmonton (AB): Institute ofPharmaco-Economics, 2002
Manns B, Tonelli M, Shrive F, et al. Sevelamer in patients with end-stage renal disease: a systematic review and economic evaluation. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2006 [online]. Available from URL: http://www.cadth.ca/media/pdf/HTA_349_sevelamer_tr_e.pdf [Accessed 2009 Jan 1]
Kiberd BA, Larson T. Estimating the benefits of solitary pancreas transplantation in nouremic patients with type 1 diabetes: a theoretical analysis. Transplantation 2000; 70(7): 1121–7
O’Brien JA, Patrick AR, Caro JJ. Cost of managing complications resulting from type 2 diabetes mellitus in Canada. BMC Health Services Research 2003; 3(1): 7
Ray JA, Valentine W, Secnik K, et al. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Cur Med Res Opin 2005; 21(10): 1617–29
Redekop WK, Stolk EA, Kok E, et al. Diabetic foot ulcers and amputations: estimates of health utility for use in cost-effectiveness analyses of new treatments. Diabetes Metab 2004; 30(6): 549–56
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (ULPDS 62). Med Decis Making 2002; 22(4): 340–9
Calculating the US population-based EQ-5D index score. Rockville (MD): Agency for Healthcare Research and Quality, 2005 [online]. Available from URL: http://www.ahrq.gov/rice/EQ5Dscore.htm [Accessed 2007 Oct 10]
Statistics Canada. Summary tables: diabetes, by sex, provinces and territories [online]. Available from URL: http://www40.statcan.ca [Accessed 2009 Sep 1]
Ontario Drug Benefit Plan, 2008 [online]. Available from URL: http://www.health.gov.on.ca/english/providers/program/drugs/edition_41.html [Accessed 2008 Oct 1]
Ontario Ministry of Health and Long-Term Care. Formulary search [online]. Available from URL: https://www.healthinfo.moh.gov.on.ca/formulary/index.jsp [Accessed 2008 Sep 1]
Régie de l’assurance maladie du Québec. Liste des medicaments. Québec (QC): Bibliothèque et Archives Nationales du Québec, 2008 Jun
Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the economic evaluation of health technologies: Canada. 3rd ed. Ottawa (ON): CADTH Publications, 2006 [online]. Available from URL: http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf [Accessed 2009 Jan 1]
ISPOR Global Health Care Systems Road Map. Reimbursement processes around the world [online]. Available from URL: http://www.ispor.org/HTARoadMaps [Accessed 2009 Jun 1]
National Institute for Health and Clinical Excellence (NICE). Guidance on the use of long-acting insulin analogues for the treatment of diabetes: insulin glargine [technology appraisal guidance no. 53]. London: NICE, 2002 Jan 12 [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/53_Insulin_analogues_full_guidance.pdf [Accessed 2009 Sep 1]
Currie CJ, Morrissey M, Peters JR, et al. The impact of health-related quality of life (EQ-5 index) in people with type 1 diabetes who experience severe hypoglycaemia [abstract]. Diabetologia 2005; 48: A292–3
Halpern EF, Weinstein MC, Hunink MG, et al. Representing both first- and second-order uncertainties by Monte Carlo simulation for groups of patients. Med Decis Making 2000; 20: 314–22
Briggs AH, Gray AM. Handling uncertainty in economic evaluations of healthcare interventions. BMJ 1999; 319(7210): 635–8
Fenwick E, Briggs A. Cost-effectiveness acceptability curves in the dock: case not proven? Med Decis Making 2007; 27(2): 93–5
Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006; 22(8): 1523–34
Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and 2 diabetes in Canada. Pharmacoeconomics 2007; 25(3): 253–66
Pelletier EM, Smith P, Boye KS, et al. Direct medical costs for type 2 diabetes mellitus complications in the US commercial payer setting: a resource for economic research. Appl Health Econ Health Policy 2008; 6(2): 103–12
Acknowledgements
Funding for the study and manuscript preparation was provided by sanofi-aventis, Canada.
The sponsor had no role in the conduct of the cost-effectiveness analyses. The sponsor did provide editorial assistance for the manuscript. Although results, interpretations and conclusions were not dictated by the sponsor, the paper did receive sponsor approval prior to submission.
Sandra Tunis is a full-time employee of IMS Health Inc., which received funding from the study sponsor in the form of a research and consulting contract. Luc Sauriol is a full-time employee of, and owns stock options in, sanofi-aventis, the manufacturer of insulin glargine. Michael Minshall was a full-time employee of IMS Health while this study was conducted and is now at Covidien, North Haven, CT, USA.
The authors wish to thank Meaghan St. Charles for her contribution to an earlier iteration of the cost-effectiveness analysis.
A portion of this work, representing an earlier version of the analyses, was presented at the International Society for Pharmacoeconomics and Outcomes Research 11th Annual European Congress; 2008 Nov 8–11; Athens, Greece.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tunis, S.L., Sauriol, L. & Minshall, M.E. Cost effectiveness of insulin glargine plus oral antidiabetes drugs compared with premixed insulin alone in patients with type 2 diabetes mellitus in Canada. Appl Health Econ Health Policy 8, 267–280 (2010). https://doi.org/10.2165/11535380-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11535380-000000000-00000