Abstract
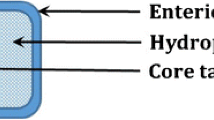
Most therapeutic peptides and proteins are administered via the parenteral route, which presents numerous drawbacks and limitations. To overcome these drawbacks, alternative administration routes, such as oral or mucosal routes, have been investigated. The oral route presents a series of attractive advantages for the administration of therapeutic compounds, such as the avoidance of the pain and discomfort associated with injections, good patient compliance, and being less expensive to produce. However, oral administration of peptides, proteins, or nucleic acids also presents several difficulties because of their instability in the upper gastrointestinal (GI) tract and their poor transport across biologic membranes. Among the various approaches developed to improve the oral delivery of peptides, proteins, or nucleic acids, specific delivery to the colon has attracted a lot of interest because of its potential for the local treatment of colonic diseases, systemic delivery of poorly absorbed drugs, and vaccine delivery. Numerous pharmaceutical approaches described in this review have been exploited for the development of colon-targeted drug delivery systems using various concepts, such as pH-dependent, time-dependent, pressure-controlled, or bacterially triggered delivery systems. The action of the pH-dependent delivery systems is based on pH differences between the stomach and the ileum. Time-dependent delivery systems are based on the transit time of pharmaceutical dosage forms in the GI tract, drug release being delayed until they reach the colon. A combination of pH- and time-dependent delivery systems has also been described to avoid the drawbacks of both strategies. The pressure-controlled delivery concept exploits the physiologic luminal pressure of the colon as the driving force for site-specific delivery of drugs. Finally, bacterially triggered delivery systems exploit the enormous diversity of enzymatic activity associated with the colonic microflora. Bacterially triggered delivery systems are generally composed of polymers, which are specifically degraded by colonic enzymes of microbial origin. These polymers have been used to form prodrugs with the drug moiety, as coating materials for the drug core, or as embedding media to entrap the drug into matrix or hydrogel systems. Each of these concepts has advantages and limitations. They present varied colonic specificity and, among them, bacterially triggered delivery systems in particular show the greatest potential for colonic delivery of peptides, proteins, and nucleic acids.










Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Lambkin I, Pinilla C. Targeting approaches to oral drug delivery. Expert Opin Biol Ther 2002 Jan; 2(1): 67–73
Shah RB, Ahsan F, Khan MA. Oral delivery of proteins: progress and prognostication. Crit Rev Ther Drug Carrier Syst 2002; 19(2): 135–69
Lee HJ. Protein drug oral delivery: the recent progress. Arch Pharm Res 2002 Oct; 25(5): 572–84
Torres-Lugo M, Peppas NA. Transmucosal delivery systems for calcitonin: a review. Biomaterials 2000 Jun; 21(12): 1191–6
Nakase H, Okazaki K, Tabata Y, et al. New cytokine delivery system using gelatin microspheres containing interleukin-10 for experimental inflammatory bowel disease. J Pharmacol Exp Ther 2002 Apr; 301(1): 59–65
Lindsay JO, Ciesielski CJ, Scheinin T, et al. Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut 2003 Jul; 52(7): 981–7
Liu L, Sakaguchi T, Kanda T, et al. Delivery of interleukin-12 in gelatin hydrogels effectively suppresses development of transplanted colonal carcinoma in mice. Cancer Chemother Pharmacol 2003 Jan; 51(1): 53–7
Zheng S, Xiao ZX, Pan YL, et al. Continuous release of interleukin 12 from microencapsulated engineered cells for colon cancer therapy. World J Gastroenterol 2003 May; 9(5): 951–5
McCormick F. Signaling networks that cause cancer. Trends Genet 1999 Dec; 15(12): M53–6
Midgley R, Kerr D. Colorectal cancer. Lancet 1999 Jan; 353(9150): 391–9
Pauletti GM, Jeffrey A, Siahaan TJ, et al. Improvement of oral peptide bioavailability: peptidomimetics and prodrug strategies. Adv Drug Deliv Rev 1997 Sep; 27(2–3): 235–56
Lee VHL, Yamamoto A. Penetration and enzymatic barriers to peptide and protein absorption. Adv Drug Deliv Rev 1989 Oct-Dec; 4(2): 171–207
Woodley JF. Enzymatic barriers for GI peptide and protein delivery. Crit Rev Ther Drug Carrier Syst 1994; 11(2–3): 61–95
Humphrey MJ, Ringrose PS. Peptides and related drugs: a review of their absorption, metabolism, and excretion. Drug Metab Rev 1986; 17(3–4): 283–310
Gonnella PA, Allan Walker W. Macromolecular absorption in the gastrointestinal tract. Adv Drug Deliv Rev 1988 Sep; 1(3): 235–48
Dice JF. Selective degradation of cytosolic proteins by lysosomes. Ann N Y Acad Sci 1992 Dec; 674: 58–64
Ho NFH, Day JS, Barsuhn CL, et al. Biophysical model approaches to mechanistic transepithelial studies of peptides. J Control Release 1990 Jan; 11(1–3): 3–24
Hovgaard L, Mack EJ, Kim SW. Insulin stabilization and GI absorption. J Control Release 1992 Mar; 19(1–3): 99–108
Merkle HP. New aspects of pharmaceutical dosage forms for controlled drug delivery of peptides and proteins. Eur J Pharm Sci 1994 Sep; 2(1–2): 19–21
Brayden DJ, O’Mahony DJ. Novel oral drug delivery gateways for biotechnology products: polypeptides and vaccines. Pharm Sci Technol Today 1998 Oct; 1(7): 291–9
Vyas SP, Venugopalan P, Sood A, et al. Some approaches to improve bioavailability of peptides and proteins through oral and other mucosal routes. Pharmazie 1997 May; 52(5): 339–45
Mackay M, Phillips J, Hastewell J. Peptide drug delivery: colonic and rectal absorption. Adv Drug Deliv Rev 1997 Nov; 28(2): 253–73
Rubinstein A, Tirosh B, Baluom M, et al. The rationale for peptide drug delivery to the colon and the potential of polymeric carriers as effective tools. J Control Release 1997 May; 46(1–2): 59–73
Zheng Y, Qiu Y, Lu MF, et al. Permeability and absorption of leuprolide from various intestinal regions in rabbits and rats. Int J Pharm 1999 Aug; 185(1): 83–92
Uchiyama T, Sugiyama T, Quan YS, et al. Enhanced permeability of insulin across the rat intestinal membrane by various absorption enhancers: their intestinal mucosal toxicity and absorption-enhancing mechanism of n-lauryl-beta-D-maltopyranoside. J Pharm Pharmacol 1999 Nov; 51(11): 1241–50
Haupt S, Rubinstein A. The colon as a possible target for orally administered peptide and protein drugs. Crit Rev Ther Drug Carrier Syst 2002; 19(6): 499–551
Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology 1984 Jan; 86(1): 174–93
Watts PJ, Illum L. Colonic drug delivery. Drug Dev Ind Pharm 1997; 29(9): 893–913
Kinget R, Kalala W, Vervoort L, et al. Colonic drug targeting. J Drug Target 1998; 6(2): 129–49
Evans DF, Pye G, Bramley R, et al. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988 Aug; 29(8): 1035–41
Shareef M, Khar R, Ahuja A, et al. Colonic drug delivery: an updated review. AAPS PharmSci 2003; 5(2): 161–86
David A, Yagen B, Sintov A, et al. Acrylic polymers for colon-specific drug delivery. STP Pharma Sci 1997; 7(6): 546–54
Ashford M, Fell JT, Attwood D, et al. An in vitro investigation into the suitability of pH-dependent polymers for colonic targeting. Int J Pharm 1993 Apr; 91(2–3): 241–5
Ashford M, Fell JT, Attwood D, et al. An in vivo investigation into the suitability of pH dependent polymers for colonic targeting. Int J Pharm 1993 Jun; 95(1–3): 193–9
Cole ET, Scott RA, Connor AL, et al. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm 2002 Jan; 231(1): 83–95
Gupta VK, Beckert TE, Price JC. A novel pH- and time-based multi-unit potential colonic drug delivery system: I. Development. Int J Pharm 2001 Feb; 213(1–2): 83–91
Goto T, Tanida N, Yoshinaga T, et al. Pharmaceutical design of a novel colontargeted delivery system using two-layer-coated tablets of three different pharmaceutical formulations, supported by clinical evidence in humans. J Control Release 2004 May; 97(1): 31–42
Marvola M, Nykanen P, Rautio S, et al. Enteric polymers as binders and coating materials in multiple-unit site-specific drug delivery systems. Eur J Pharm Sci 1999 Feb; 7(3): 259–67
Nykanen P, Krogars K, Sakkinen M, et al. Organic acids as excipients in matrix granules for colon-specific drug delivery. Int J Pharm 1999 Jul; 184(2): 251–61
Nykanen P, Lempaa S, Aaltonen ML, et al. Citric acid as excipient in multiple-unit enteric-coated tablets for targeting drugs on the colon. Int J Pharm 2001 Oct; 229(1–2): 155–62
Nykanen P, Sten T, Jurjenson H, et al. Citric acid as a pH-regulating additive in granules and the tablet matrix in enteric-coated formulations for colon-specific drug delivery. Pharmazie 2004 Apr; 59(4): 268–73
Rubinstein A. Microbially controlled drug delivery to the colon. Biopharm Drug Dispos 1990 Aug-Sep; 11(6): 465–75
Davis SS, Hardy JG, Fara JW. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986 Aug; 27(8): 886–92
MacNeil ME, Rachid A, Stevens HNE. Dispensing device. Patent WO 9009168. (1990)
Binns JS, Stevens HNE, Bakhshaee M, et al. Colon targeted release using the Pulsincap delivery system. Proceedings of the 21st International Symposium on Controlled Release of Bioactive Materials Controlled Release Society, Nice, France 1994; 21: 260–1
Binns J, Stevens HNE, McEwen J, et al. The tolerability of multiple oral doses of Pulsincap™ capsules in healthy volunteers. J Control Release 1996 Feb; 38(2–3): 151–8
Gazzaniga A, Iamartino P, Maffione G, et al. Oral delayed-release system for colonic specific delivery. Int J Pharm 1994 Jul; 108(1): 77–83
Pozzi F, Furlani P, Gazzaniga A, et al. The Time Clock system: a new oral dosage form for fast and complete release of drug after predetermined lag time. J Control Release 1994 Aug; 31(1): 99–108
Fukui E, Miyamura N, Uemura K, et al. Preparation of enteric coated timed-release press-coated tablets and evaluation of their function by in vitro and in vivo tests for colon targeting. Int J Pharm 2000 Aug; 204(1–2): 7–15
Fukui E, Miyamura N, Kobayashi M. An in vitro investigation of the suitability of press-coated tablets with hydroxypropylmethylcellulose acetate succinate (HPMCAS) and hydrophobic additives in the outer shell for colon targeting. J Control Release 2001 Jan; 70(1–2): 97–107
Peerapattana J, Otsuka K, Otsuka M. Time-controlled pulse-drug release from dry-coated wax matrix tablets for colon drug delivery. Biomed Mater Eng 2004; 14(3): 293–301
Adkin DA, Davis SS, Sparrow RA, et al. Colonic transit of different sized tablets in healthy subjects. J Control Release 1993 Feb; 23(2): 147–56
Ishibashi T, Hatano H, Kobayashi M, et al. Design and evaluation of a new capsule-type dosage form for colon-targeted delivery of drugs. Int J Pharm 1998 Jun; 168(1): 31–40
Ishibashi T, Hatano H, Kobayashi M, et al. In vivo drug release behavior in dogs from a new colon-targeted delivery system. J Control Release 1999 Jan; 57(1): 45–53
Gupta VK, Beckert TE, Price JC. Development of a novel multi-particulate colonic delivery system using multi-functional coatings of aqueous polymethacrylates. 3rd World Meeting (APV/APGI) on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology; Berlin: APGI/APV, 2000: 875–6
Gupta VK, Assmus MW, Beckert TE, et al. A novel pH- and time-based multi-unit potential colonic drug delivery system: II. Optimization of multiple response variables. Int J Pharm 2001 Feb; 213(1–2): 93–102
Bott C, Rudolph MW, Schneider AR, et al. In vivo evaluation of a novel pH- and time-based multiunit colonic drug delivery system. Aliment Pharmacol Ther 2004 Aug; 20(3): 347–53
Muraoka M, Hu Z, Shimokawa T, et al. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J Control Release 1998 Mar; 52(1–2): 119–29
Hu Z, Kimura G, Mawatari S-S, et al. New preparation method of intestinal pressure-controlled colon delivery capsules by coating machine and evaluation in beagle dogs. J Control Release 1998 Dec; 56(1–3): 293–302
Jeong Y-I, Ohno T, Hu Z, et al. Evaluation of an intestinal pressure-controlled colon delivery capsules prepared by a dipping method. J Control Release 2001 Apr; 71(2): 175–82
Drasar BS, Shiner M, McLeod GM. Studies on the intestinal flora: I. The bacterial flora of the gastrointestinal tract in healthy and achlorhydric persons. Gastroenterology 1969 Jan; 56(1): 71–9
Gorbach SL, Nahas L, Lerner PI, et al. Studies of intestinal microflora: I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology 1967 Dec; 53(6): 845–55
Hill MJ, Drasar BS. The normal colonic bacterial flora. Gut 1975 Apr; 16(4): 318–23
Moore WEC, Holdeman LV. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol 1974 May; 27(5): 961–79
Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987 Oct; 28(10): 1221–7
Scheline RR. Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol Rev 1973 Dec; 25(4): 451–523
Azad Khan AK, Piris J, Truelove SC. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet 1977 Oct; II(8044): 892–5
Peppercorn MA. Sulfasalazine: pharmacology, clinical use, toxicity, and related new drug development. Ann Intern Med 1984 Sep; 101(3): 377–86
Chan RP, Pope DJ, Gilbert AP, et al. Studies of two novel sulfasalazine analogs, ipsalazide and balsalazide. Dig Dis Sci 1983 Jul; 28(7): 609–15
Van Hogezand RA. Pharmacokinetics of olsalazine and its metabolites. Scand J Gastroenterol Suppl 1988; 148: 17–20
Willoughby CP, Aronson JK, Agback H, et al. Distribution and metabolism in healthy volunteers of disodium azodisalicylate, a potential therapeutic agent for ulcerative colitis. Gut 1982 Dec; 23(12): 1081–7
Brown JP, McGarraugh GV, Parkinson TM, et al. A polymeric drug for treatment of inflammatory bowel disease. J Med Chem 1983 Sep; 26(9): 1300–7
Wiwattanapatapee R, Lomlim L, Saramunee K. Dendrimers conjugates for colonic delivery of 5-aminosalicylic acid. J Control Release 2003 Feb; 88(1): 1–9
Wiwattanapatapee R, Carreno-Gomez B, Malik N, et al. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm Res 2000 Aug; 17(8): 991–8
Sakuma S, Lu ZR, Kopeckova P, et al. Biorecognizable HPMA co-polymer-drug conjugates for colon-specific delivery of 9-aminocamptothecin. J Control Release 2001 Aug; 75(3): 365–79
Friend DR, Chang GW. Drug glycosides: potential prodrugs for colon-specific drug delivery. J Med Chem 1985 Jan; 28(1): 51–7
Friend DR, Chang GW. A colon-specific drug-delivery system based on drug glycosides and the glycosidases of colonic bacteria. J Med Chem 1984 Mar; 27(3): 261–6
Friend DR, Tozer TN. Drug glycosides in oral colon-specific drug delivery. J Control Release 1992 Mar; 19(1–3): 109–20
Simpkins JW, Smulkowski M, Dixon R, et al. Evidence for the delivery of narcotic antagonists to the colon as their glucuronide conjugates. J Pharmacol Exp Ther 1988 Jan; 244(1): 195–205
Haeberlin B, Rubas W, Nolen HW, et al. In vitro evaluation of dexamethasone-beta-D-glucuronide for colon-specific drug delivery. Pharm Res 1993 Nov; 10(11): 1553–62
Cui N, Friend DR, Fedorak RN. A budesonide prodrug accelerates treatment of colitis in rats. Gut 1994 Oct; 35(10): 1439–46
Harboe E, Larsen C, Johansen M, et al. Macromolecular prodrugs: XV. Colontargeted delivery: bioavailability of naproxen from orally administered dextrannaproxen ester prodrugs varying in molecular size in the pig. Pharm Res 1989 Nov; 6(11): 919–23
Larsen C, Harboe E, Johansen M, et al. Macromolecular prodrugs: XVI. Colontargeted delivery: comparison of the rate of release of naproxen from dextran ester prodrugs in homogenates of various segments of the pig gastrointestinal (GI) tract. Pharm Res 1989 Dec; 6(12): 995–9
Larsen C, Jensen BH, Olesen HP. Stability of ketoprofen-dextran ester prodrugs in homogenates of various segments of the pig GI tract. Acta Pharm Nord 1991; 3(1): 41–4
Larsen C, Jensen BH, Olesen HP. Bioavailability of ketoprofen from orally administered ketoprofen-dextran ester prodrugs in the pig. Acta Pharm Nord 1991; 3(2): 71–6
McLeod AD, Friend DR, Tozer TN. Synthesis and chemical stability of glucocorticoid-dextran esters: potential prodrugs for colon-specific delivery. Int J Pharm 1993 May; 92(1–3): 105–14
McLeod AD, Friend DR, Tozer TN. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: hydrolysis in rat gastrointestinal tract contents. J Pharm Sci 1994 Sep; 83(9): 1284–8
McLeod AD, Tolentino L, Tozer TN. Glucocorticoid-dextran conjugates as potential prodrugs for colon-specific delivery: steady-state pharmacokinetics in the rat. Biopharm Drug Dispos 1994 Mar; 15(2): 151–61
McLeod AD, Fedorak RN, Friend DR, et al. A glucocorticoid prodrug facilitates normal mucosal function in rat colitis without adrenal suppression. Gastroenterology 1994 Feb; 106(2): 405–13
Hirayama F, Minami K, Uekama K. In-vitro evaluation of Biphenylyl Acetic Acid-β-Cyclodextrin conjugates as colon-targeting prodrugs: drug release behavior in rat biological media. J Pharm Pharmacol 1996 Jan; 48(1): 27–31
Uekama K, Minami K, Hirayama F. 6A-O-[(4-biphenylyl)acetyl]-alpha-, -beta-, and -gamma-cyclodextrins and 6A-deoxy-6A-[[(4-biphenylyl)acetyl]amino]-alpha-, -beta-, and -gamma-cyclodextrins: potential prodrugs for colon-specific delivery. J Med Chem 1997 Aug; 40(17): 2755–61
Minami K, Hirayama F, Uekama K. Colon-specific drug delivery based on a cyclodextrin prodrug: release behavior of biphenylylacetic acid from its cyclodextrin conjugates in rat intestinal tracts after oral administration. J Pharm Sci 1998 Jun; 87(6): 715–20
Saffran M, Kumar GS, Savariar C, et al. A new approach to the oral administration of insulin and other peptide drugs. Science 1986 Sep; 233(4768): 1081–4
Saffran M, Field JB, Pena J, et al. Oral insulin in diabetic dogs. J Endocrinol 1991 Nov; 131(2): 267–78
Van den Mooter G, Samyn C, Kinget R. Azo polymers for colon-specific drug delivery. Int J Pharm 1992 Nov; 87(1–3): 37–46
Van den Mooter G, Samyn C, Kinget R. Azo polymers for colon-specific drug delivery: II. Influence of the type of azo polymer on the degradation by intestinal microflora. Int J Pharm 1993 Aug; 97(1–3): 133–9
Brondsted H, Kopecek J. Hydrogels for site-specific oral drug delivery: synthesis and characterization. Biomaterials 1991 Aug; 12(6): 584–92
Brondsted H, Kopecek J. Hydrogels for site-specific drug delivery to the colon: in vitro and in vivo degradation. Pharm Res 1992 Dec; 9(12): 1540–5
Yang H, Cao SG, Ma L, et al. A new kind of immobilized lipase in organic solvent and its structure model. Biochem Biophys Res Commun 1994 Apr; 200(1): 83–8
Van den Mooter G, Samyn C, Kinget R. Characterization of colon-specific azo polymers: a study of the swelling properties and the permeability of isolated polymer films. Int J Pharm 1994 Oct; 111(2): 127–36
Van den Mooter G, Samyn C, Kinget R. The relation between swelling properties and enzymatic degradation of azo polymers designed for colon-specific drug delivery. Pharm Res 1994 Dec; 11(12): 1737–41
Van den Mooter G, Samyn C, Kinget R. In vivo evaluation of a colon-specific drug delivery system: an absorption study of theophylline from capsules coated with azo polymers in rats. Pharm Res 1995 Feb; 12(2): 244–7
Kimura Y, Makita Y, Kumagai T, et al. Degradation of azo-containing poly-urethane by the action of intestinal flora: its mechanism and application as a drug delivery system. Polymer 1992; 33(24): 5294–9
Schacht E, Gevaert A, Kenawy ER, et al. Polymers for colon specific drug delivery. J Control Release 1996 May; 39(2–3): 327–38
Lloyd AW, Martin GP, Soozandehfar SH. Azopolymers: a means of colon specific drug delivery? Int J Pharm 1994 Jun; 106(3): 255–60
Bragger JL, Lloyd AW, Soozandehfar SH, et al. Investigations into the azo reducing activity of a common colonic microorganism. Int J Pharm 1997 Nov; 157(1): 61–71
Semde R, Pierre D, Geuskens G, et al. Study of some important factors involved in azo derivative reduction by Clostridium perfringens. Int J Pharm 1998 Feb; 161(1): 45–54
Hovgaard L, Brondsted H. Current applications of polysaccharides in colon targeting. Crit Rev Ther Drug Carrier Syst 1996; 13(3–4): 185–223
Milojevic S, Newton JM, Cummings JH, et al. Amylose as a coating for drug delivery to the colon: preparation and in vitro evaluation using 5-aminosalicylic acid pellets. J Control Release 1996 Jan; 38(1): 75–84
Milojevic S, Newton JM, Cummings JH, et al. Amylose as a coating for drug delivery to the colon: preparation and in vitro evaluation using glucose pellets. J Control Release 1996 Jan; 38(1): 85–94
Cummings JH, Milojevic S, Harding M, et al. In vivo studies of amylose- and ethylcellulose-coated [13C]glucose microspheres as a model for drug delivery to the colon. J Control Release 1996 Jun; 40(1–2): 123–31
Tozaki H, Komoike J, Tada C, et al. Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci 1997 Sep; 86(9): 1016–21
Zhang H, Alsarra IA, Neau SH. An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int J Pharm 2002 Jun; 239(1–2): 197–205
Aiedeh K, Taha MO. Synthesis of chitosan succinate and chitosan phthalate and their evaluation as suggested matrices in orally administered, colon-specific drug delivery systems. Arch Pharm (Weinheim) 1999 Mar; 332(3): 103–7
Rubinstein A, Nakar D, Sintov A. Chondroitin sulfate: a potential biodegradable carrier for colon-specific drug delivery. Int J Pharm 1992 Jul; 84(2): 141–50
Siefke V, Weckenmann HP, Bauer KH. [beta]-cyclodextrin matrix films for colon-specific drug delivery. Proceedings of the 20th International symposium on Controlled Release Society. Washington (DC): Controlled Release Society, 1993: 3
Hirsch S, Binder V, Kolter K, et al. Lauroyldextran as a coating material for site-specific drug delivery to the colon: in vitro dissolution of coated tablets. Proceedings of the 24th International Symposium on Controlled Release of Bioactive Materials,. Stockholm: Controlled Release Society, 1997: 80
Lauroyldextran and crosslinked galactomannan as coating materials for site-specific drug delivery to the colon: in vitro dissolution of coated tablets. Proceedings of the 24th International Symposium on Controlled Release of Bioactive Materials, Stockholm, Sweden, 1997; 24: 80
Krishnaiah YSR, Satyanarayana S, Rama Prasad YV, et al. Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm 1998 Sep; 171(2): 137–46
Krishnaiah YSR, Satyanarayana S, Prasad YV. Studies of guar gum compression-coated 5-aminosalicylic acid tablets for colon-specific drug delivery. Drug Dev Ind Pharm 1999 May; 25(5): 651–7
Krishnaiah YSR, Indira Muzib Y, Bhaskar P. In vivo evaluation of guar gum-based colon-targeted drug delivery systems of ornidazole in healthy human volunteers. J Drug Target 2003 Feb; 11(2): 109–15
Wong D, Larrabee S, Clifford K, et al. USP dissolution apparatus III (reciprocating cylinder) for screening of guar-based colonic delivery formulations. J Control Release 1997 Aug; 47(2): 173–9
Kenyon CJ, Nardi RV, Wong D, et al. Colonic delivery of dexamethasone: a pharmacoscintigraphic evaluation. Aliment Pharmacol Ther 1997 Feb; 11(1): 205–13
Krishnaiah YSR, Veer Raju P, Dinesh Kumar B, et al. Development of colon targeted drug delivery systems for mebendazole. J Control Release 2001 Nov; 77(1–2): 87–95
Krishnaiah YSR, Veer Raju P, Dinesh Kumar B, et al. Pharmacokinetic evaluation of guar gum-based colon-targeted drug delivery systems of mebendazole in healthy volunteers. J Control Release 2003 Feb; 88(1): 95–103
Vervoort L, Kinget R. In vitro degradation by colonic bacteria of inulinHP incorporated in Eudragit RS films. Int J Pharm 1996 Mar; 129(1–2): 185–90
Ashford M, Fell JT, Attwood D, et al. An evaluation of pectin as a carrier for drug targeting to the colon. J Control Release 1993 Sep; 26(3): 213–20
Wakerly Z, Fell JT, Attwood D, et al. Pectin/ethylcellulose film coating formulations for colonic drug delivery. Pharm Res 1996 Aug; 13(8): 1210–2
Semde R, Amighi K, Devleeschouwer MJ, et al. Studies of pectin HM/Eudragit RL/Eudragit NE film-coating formulations intended for colonic drug delivery. Int J Pharm 2000 Mar; 197(1–2): 181–92
Fernandez-Hervas MJ, Fell JT. Pectin/chitosan mixtures as coatings for colon-specific drug delivery: an in vitro evaluation. Int J Pharm 1998 Jun; 169(1): 115–9
Macleod GS, Fell JT, Collett JH, et al. Selective drug delivery to the colon using pectin: hydroxypropyl methylcellulose film coated tablet. Int J Pharm 1999 Oct; 187(2): 251–7
Macleod GS, Collett JH, Fell JT. The potential use of mixed films of pectin, chitosan and HPMC for bimodal drug release. J Control Release 1999 Apr; 58(3): 303–10
Turkoglu M, Ugurlu T. In vitro evaluation of pectin-HPMC compression coated 5-aminosalicylic acid tablets for colonic delivery. Eur J Pharm Biopharm 2002 Jan; 53(1): 65–73
Rubinstein A, Radai R, Ezra M, et al. In vitro evaluation of calcium pectinate: a potential colon-specific drug delivery carrier. Pharm Res 1993 Feb; 10(2): 258–63
Wakerly Z, Fell J, Attwood D, et al. Studies on amidated pectins as potential carriers in colonic drug delivery. J Pharm Pharmacol 1997 Jun; 49(6): 622–5
Adkin DA, Kenyon CJ, Lerner EI, et al. The use of scintigraphy to provide “proof of concept” for novel polysaccharide preparations designed for colonic drug delivery. Pharm Res 1997 Jan; 14(1): 103–7
Munjeri O, Collett JH, Fell JT. Amidated pectin hydrogel beads for colonic drug de livery: an in vitro study. Drug Deliv 1997 Sep; 4(3): 207–11
Munjeri O, Collett JH, Fell JT. Hydrogel beads based on amidated pectins for colon-specific drug delivery: the role of chitosan in modifying drug release. J Control Release 1997 Jun; 46(3): 273–8
Kim TH, Park YH, Kim KJ, et al. Release of albumin from chitosan-coated pectin beads in vitro. Int J Pharm 2003 Jan; 250(2): 371–83
Sriamornsak P, Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. preparation and in vitro release studies. Int J Pharm 1998 Jan; 160(2): 207–12
Sriamornsak P. Investigation on pectin as a carrier for oral delivery of proteins using calcium pectinate gel beads. Int J Pharm 1998 Jul; 169(2): 213–20
Musabayane CT, Munjeri O, Bwititi P, et al. Orally administered, insulin-loaded amidated pectin hydrogel beads sustain plasma concentrations of insulin in streptozotocin-diabetic rats. J Endocrinol 2000 Jan; 164(1): 1–6
El-Gibaly I. Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int J Pharm 2002 Jan; 232(1–2): 199–211
Leloup VM, Colonna P, Ring SG. [Alpha]-amylase adsorption on starch crystallites. Biotechnol Bioeng 1991; 38(2): 127–34
Ring SG, Gee JM, Whittam M, et al. Resistant starch: its chemical form in foodstuffs and effect on digestibility in vitro. Food Chem 1988; 28(2): 97–109
Siew LF, Basit AW, Newton JM. The properties of amylose-ethylcellulose films cast from organic-based solvents as potential coatings for colonic drug delivery. Eur J Pharm Sci 2000 Aug; 11(2): 133–9
Siew LF, Basit AW, Newton JM. The potential of organic-based amylose-ethylcellulose film coatings as oral colon-specific drug delivery systems [abstract]. AAPS PharmSciTech 2000 Jul; 1(3): E22
Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm 1998 Nov; 24(11): 979–93
Lorenzo-Lamosa ML, Remunan-Lopez C, Vila-Jato JL, et al. Design of microencapsulated chitosan microspheres for colonic drug delivery. J Control Release 1998 Mar; 52(1–2): 109–18
Orienti I, Cerchiara T, Luppi B, et al. Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int J Pharm 2002 May; 238(1–2): 51–9
Zambito Y, Di Colo G. Preparation and in vitro evaluation of chitosan matrices for colonic controlled drug delivery. J Pharm Pharm Sci 2003 May-Aug; 6(2): 274–81
Chourasia MK, Jain SK. Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv 2004 May-Jun; 11(3): 201–7
Salyers AA. Energy sources of major intestinal fermentative anaerobes. Am J Clin Nutr 1979 Jan; 32(1): 158–63
Salyers AA, O’Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol 1980 Aug; 143(2): 772–80
Rubinstein A, Nakar D, Sintov A. Colonic drug delivery: enhanced release of indomethacin from cross-linked chondroitin matrix in rat cecal content. Pharm Res 1992 Feb; 9(2): 276–8
Sintov A, Di-Capua N, Rubinstein A. Cross-linked chondroitin sulphate: characterization for drug delivery purposes. Biomaterials 1995 Apr; 16(6): 473–8
Fetzner A, Bohm S, Schreder S, et al. Degradation of raw or film-incorporated [beta]-cyclodextrin by enzymes and colonic bacteria. Eur J Pharm Biopharm 2004 Jul; 58(1): 91–7
Bauer KH, Kesselhut JF. Novel pharmaceutical excipients for colon targeting. STP Pharma Sci 1995; 5(1): 54–9
Cheetham NWH, Mashimba ENM. Conformational aspects of xanthan-galactomannan gelation: further evidence from optical-rotation studies. Carbohyd Polym 1991; 14(1): 17–27
Jain NK, Kulkarni K, Talwar N. Controlled-release tablet formulation of isoniazid. Pharmazie 1992 Apr; 47(4): 277–8
Macfarlane GT, Hay S, Macfarlane S, et al. Effect of different carbohydrates on growth, polysaccharidase and glycosidase production by Bacteroides ovatus, in batch and continuous culture. J Appl Bacteriol 1990 Feb; 68(2): 179–87
Prasad YV, Krishnaiah YSR, Satyanarayana S. In vitro evaluation of guargum as a carrier for colon-specific drug delivery. J Control Release 1998 Feb; 51(2–3): 281–7
Krishnaiah YSR, Satyanarayana S, Rama Prasad YV, et al. Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy human volunteers. J Control Release 1998 Nov; 55(2–3): 245–52
Tugcu-Demiroz F, Acarturk F, Takka S, et al. In-vitro and in-vivo evaluation of mesalazine-guar gum matrix tablets for colonic drug delivery. J Drug Target 2004 Feb; 12(2): 105–12
Katsuma M, Watanabe S, Kawai H, et al. Studies on lactulose formulations for colon-specific drug delivery. Int J Pharm 2002 Dec; 249(1–2): 33–43
Morris ER, Powell DA, Gidley MJ, et al. Conformations and interactions of pectins: I. polymorphism between gel and solid states of calcium polygalacturonate. J Mol Biol 1982 Mar; 155(4): 507–16
Ashford M, Fell J, Attwood D, et al. Studies on pectin formulations for colonic drug delivery. J Control Release 1994; 30: 225–32
Macleod GS, Fell JT, Collett JH. Studies on the physical properties of mixed pectin/ethylcellulose films intended for colonic drug delivery. Int J Pharm 1997 Nov; 157(1): 53–60
Semde R, Amighi K, Pierre D, et al. Leaching of pectin from mixed pectin/insoluble polymers films intended for colonic drug delivery. Int J Pharm 1998 Nov; 174(1–2): 233–41
Semde R, Amighi K, Devleeschouwer MJ, et al. Effect of pectinolytic enzymes on the theophylline release from pellets coated with water insoluble polymers containing pectin HM or calcium pectinate. Int J Pharm 2000 Mar; 197(1–2): 169–79
Meshali MM, Gabr KE. Effect of interpolymer complex formation of chitosan with pectin or acacia on the release behaviour of chlorpromazine HC1. Int J Pharm 1993 Feb; 89(3): 177–81
Ofori-Kwakye K, Fell JT. Biphasic drug release: the permeability of films containing pectin, chitosan and HPMC. Int J Pharm 2001 Sep; 226(1–2): 139–45
Ofori-Kwakye K, Fell JT. Biphasic drug release from film-coated tablets. Int J Pharm 2003 Jan; 250(2): 431–40
Ofori-Kwakye K, Fell JT, Sharma HL, et al. Gamma scintigraphic evaluation of film-coated tablets intended for colonic or biphasic release. Int J Pharm 2004 Feb; 270(1–2): 307–13
Hardy JG, Wilson CG, Wood E. Drug delivery to the proximal colon. J Pharm Pharmacol 1985 Dec; 37(12): 874–7
Ayděn Z, Akbuga J. Preparation and evaluation of pectin beads. Int J Pharm 1996; 137: 133–6
Bodmeier R, Oh K-H, Pramar Y. Preparation and evaluation of drug-containing chitosan beads. Drug Dev Ind Pharm 1989; 15(9): 1475–94
Touitou E, Rubinstein A. Targeted enterai delivery of insulin to rats. Int J Pharm 1986 Jun; 30(2–3): 95–9
Saffran M, Bedra C, Kumar GS, et al. Vasopressin: a model for the study of effects of additives on the oral and rectal administration of peptide drugs. J Pharm Sci 1988 Jan; 77(1): 33–8
Tozaki H, Nishioka J, Komoike J, et al. Enhanced absorption of insulin and (Asu(l,7))eel-calcitonin using novel azopolymer-coated pellets for colon-specific drug delivery. J Pharm Sci 2001 Jan; 90(1): 89–97
Xing L, Dawei C, Liping X, et al. Oral colon-specific drug delivery for bee venom peptide: development of a coated calcium alginate gel beads-entrapped liposome. J Control Release 2003 Dec; 93(3): 293–300
Sandberg JW, Lau C, Jacomino M, et al. Improving access to intestinal stem cells as a step toward intestinal gene transfer. Hum Gene Ther 1994 Mar; 5(3): 323–9
Lau C, Soriano HE, Ledley FD, et al. Retroviral gene transfer into the intestinal epithelium. Hum Gene Ther 1995 Sep; 6(9): 1145–51
Croyle MA, Stone M, Amidon GL, et al. In vitro and in vivo assessment of adenovirus 41 as a vector for gene delivery to the intestine. Gene Ther 1998 May; 5(5): 645–54
Wirtz S, Galle PR, Neurath MF. Efficient gene delivery to the inflamed colon by local administration of recombinant adenoviruses with normal or modified fibre structure. Gut 1999 Jun; 44(6): 800–7
Walter E, Croyle MA, Davidson BL, et al. Adenovirus mediated gene transfer to intestinal epithelial cells as a potential approach for oral delivery of peptides and proteins. J Control Release 1997 May; 46(1–2): 75–87
Chung-Faye GA, Kerr DJ, Young LS, et al. Gene therapy strategies for colon cancer. Mol Med Today 2000 Feb; 6(2): 82–7
Marshall E. Gene therapy death prompts review of adenovirus vector. Science 1999 Dec; 286(5448): 2244–5
Westbrook CA, Arenas RB. Gene therapy of the gut: introduction of the APC tumour-supressor gene for cancer prevention or treatment. Adv Drug Deliv Rev 1995 Dec; 17(3): 349–55
Fichera A, Guo Y, Romero L, et al. Quantitation of in vivo gene delivery by restriction enzyme PCR generated polymorphism. J Surg Res 1997 Apr; 69(1): 188–92
Lew JI, Guo Y, Kim RK, et al. Reduction of intestinal neoplasia with adenomatous polyposis coli gene replacement and COX-2 inhibition is additive. J Gastrointest Surg 2002 Jul-Aug; 6(4): 563–8
Lindahl T. Instability and decay of the primary structure of DNA. Nature 1993 Apr; 362(6422): 709–15
Sinha VR, Kumria R. Polysaccharides in colon-specific drug delivery. Int J Pharm 2001 Aug; 224(1–2): 19–38
Vandamme TF, Lenourry A, Charrueau C, et al. The use of polysaccharides to target drugs to the colon. Carbohyd Polym 2002 May; 48(3): 219–31
Alexakis T, Boadi DK, Quong D, et al. Microencapsulation of DNA within alginate microspheres and crosslinked chitosan membranes for in vivo application. Appl Biochem Biotechnol 1995 Jan; 50(1): 93–106
Quong D, Neufeld RJ. DNA protection from extracapsular nucleases, within chitosan- or poly-L-lysine-coated alginate beads. Biotechnol Bioeng 1998 Oct; 60(1): 124–34
Quong D, Yeo JN, Neufeld RJ. Stability of chitosan and poly-L-lysine membranes coating DNA-alginate beads when exposed to hydrolytic enzymes. J Microencapsul 1999 Jan-Feb; 16(1): 73–82
Padmanabhan K, Smith TJ. A preliminary investigation of modified alginates as a matrix for gene transfection in a HeLa cell model. Pharm Dev Technol 2002 Jan; 7(1): 97–101
Aggarwal N, HogenEsch H, Guo P, et al. Biodegradable alginate microspheres as a delivery system for naked DNA. Can J Vet Res 1999 Apr; 63(2): 148–52
Mittal SK, Aggarwal N, Sailaja G, et al. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine 2000 Sep; 19(2–3): 253–63
Gonzalez Ferreiro M, Tillman LG, Hardee G, et al. Alginate/poly-L-lysine microparticles for the intestinal delivery of antisense oligonucleotides. Pharm Res 2002 Jun; 19(6): 755–64
Green DW, Roh H, Pippin JA, et al. Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res 2001 Nov; 101(1): 16–20
Acknowledgments
The authors would like to acknowledge the French Ministery of Research for supporting the fellowship of Sandrine Bourgeois. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourgeois, S., Harvey, R. & Fattal, E. Polymer colon drug delivery systems and their application to peptides, proteins, and nucleic acids. Am J Drug Deliv 3, 171–204 (2005). https://doi.org/10.2165/00137696-200503030-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00137696-200503030-00003