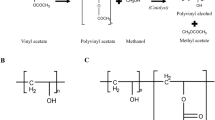
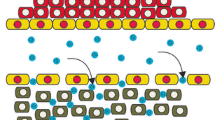
Abstract
Poly (alkylcyanoacrylate) [PACA] nanoparticles have been studied since the early 1980s as possible colloidal drug delivery systems. Several excellent general reviews have since been published on this subject. This review focuses on the use of the two different methods (encapsulation and sorption) for the entrapment of drugs and model compounds in PACA nanoparticles. The term encapsulation is used when the drug or model compound is added at the same time or before the monomer to the polymerization template. The term sorption is used when the compound is added after the polymerization has taken place. High drug entrapment can be achieved with both methods and the method of entrapment (encapsulation or sorption) should be chosen depending on the type of drug to be entrapped and the method of particle preparation (interfacial polymerization of a coarse emulsion or a microemulsion or micellar polymerization). The type, chain length, and amount of monomer used for the polymerization as well as possible interactions of the compound with the monomer during polymerization should also be considered in the choice of entrapment method and these can also influence the extent of encapsulation.










Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Fattal E, Blanco-Prieto MJ, Leo E, et al. Design of nanoparticles for vaccine delivery. In: Gander B, Merkle HP, Corradin G, editors. Antigen delivery systems: immunological and technological issues. Australia: Harwood Academic Publishers, 1997: 139–57
Couvreur P, Couarraze G, Devissaguet J, et al. Nanoparticles: preparation and characterization. In: Benita S, editor. Microencapsulation: methods and industrial applications. New York: Marcel Dekker, 1996: 183–211
Donnelly EF, Johnston DS, Pepper DC, et al. Ionic and zwitterionic polymerization of n-alkyl 2-cyanoacrylates. J Polym Sci Polym Lett Ed 1977; 15: 399–405
Kreuter J. Nanoparticles - preparation and application. In: Donbrow M, editor. Microcapsules and nanoparticles in medicine and pharmacy. Boca Raton: CRC Press, 1991: 125–48
Kreuter J. Possibilities of using nanoparticles as carriers for drugs and vaccines. J Microencapsul 1988; 5: 115–27
Mueller RH, Lherm C, Herbort J, et al. Alkylcyanoacrylate drug carriers: I. Physicochemical characterization of nanoparticles with different alkyl chain length. Int J Pharm 1992; 84: 1–11
Couvreur P, Kante B, Roland M, et al. Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: preparation, morphological and sorptive properties. J Pharm Pharmacol 1979; 31: 331–2
Muller RH, Lherm C, Herbort J, et al. In vitro model for the degradation of alkylcyanoacrylate nanoparticles. Biomaterials 1990; 11: 590–5
El-Samaligy MS, Rohdewald P, Mahmoud HA. Polyalkyl cyanoacrylate nanocapsules. J Pharm Pharmacol 1986; 38: 216–8
Henry-Michelland S, Alonso MJ, Andremont A, et al. Attachment of antibiotics to nanoparticles: preparation, drug-release and antimicrobial activity in vitro. Int J Pharm 1987; 35: 121–7
Leonard F, Kulkarni RK, Brandes G, et al. Synthesis and degradation of poly (alkyl α-cyanoacrylates). J Appl Polym Sci 1966; 10: 259–72
Lenaerts V, Couvreur P, Christiaens-Leyh D, et al. Degradation of poly (isobutyl cyanoacrylate) nanoparticles. Biomaterials 1984; 5: 65–8
Lehman K. Chemistry and application properties of polymethacrylate coating systems. In: McGinity JW, editor. Aqueous polymeric coatings for pharmaceutical dosage forms. New York: Marcel Dekker, 1997: 101–76
Couvreur P, Tulkens P, Roland M, et al. Nanocapsules: a new type of lysosomotropic carrier. FEBS Lett 1977; 84: 323–6
Al Khouri Fallouh N, Roblot-Treupel L, Fessi H, et al. Development of a new process for the manufacture of polyisobutylcyanoacrylate nanocapsules. Int J Pharm 1986; 28: 125–32
Gasco MR, Trotta M. Nanoparticles from microemulsions. Int J Pharm 1986; 29: 267–8
Watnasirichaikul S, Davies NM, Rades T, et al. Preparation of biodegradable insulin nanocapsules from biocompatible microemulsions. Pharm Res 2000; 17: 684–9
Kreuter J. Nanoparticles. In: Kreuter J, editor. Colloidal drug delivery systems. New York: Marcel Dekker, 1994: 219–342
Fresta M, Cavallaro G, Giammona G, et al. Preparation and characterization of polyethyl-2-cyanoacrylate nanocapsules containing antiepileptic drugs. Biomaterials 1996; 17: 751–8
Watnasirichaikul S, Rades T, Tucker IG, et al. Effects of formulation variables on characteristics of poly (ethylcyanoacrylate) nanocapsules prepared from w/o microemulsions. Int J Pham 2002; 235: 237–46
Soppimath KS, Aminabhavi TM, Kulkarni AR, et al. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 2001; 70: 1–20
Vauthier C, Dubernet C, Fattal E, et al. Poly (alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv Drug Deliv Rev 2003; 55: 519–48
Damge C, Michel C, Aprahamian M, et al. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes 1988; 37: 246–51
Tasset C, Barette N, Thysman S, et al. Polyisobutylcyanoacrylate nanoparticles as sustained release system for calcitonin. J Control Release 1995; 33: 23–30
Wood RW, Li VHK, Kreuter J, et al. Ocular disposition of polyhexyl-2-cyanoacrylate-(3-14C) acrylate nanoparticles in the albino rabbit. Int J Pharm 1985; 23: 175–83
Losa C, Marchai-Heussler L, Orallo F, et al. Design of new formulations for topical ocular administration: polymeric nanocapsules containing metipranolol. Pharm Res 1993; 10: 80–7
Vranckx H, Demoustier M, Deleers M. A new nanocapsule formulation with hydrophilic core: application to the oral administration of salmon calcitonin in rats. Eur J Pharm Biopharm 1996; 42: 345–7
Watnasirichaikul S, Davies NM, Rades T, et al. In-vitro release and oral bioactivity of insulin in diabetic rats using nanocapsules dispersed in biocompatible microemulsion. J Pharm Pharmacol 2002; 54: 473–80
Loebenberg R, Araujo L, Kreuter J. Body distribution of azidothymidine bound to nanoparticles after oral administration. Eur J Pharm Biopharm 1997; 44: 127–32
Kreuter J, Haenzel I. Mode of action of immunological adjuvants: some physico-chemical factors influencing the effectivity of polyacrylic adjuvants. Infect Immun 1978; 19: 667–75
Raghuvanshi RJ, Mistra A, Talwar GP, et al. Enhanced immune response with a combination of alum and biodegradable nanoparticles containing tetanus toxoid. J Microencapsul 2001; 18(6): 723–32
O’Hagan DT, Palin KJ, Davis SS. Poly(butyl-2-cyanoacrylate) particles as adjuvants for oral immunization. Vaccine 1989; 7: 213–6
Guierro I, Hernandez RM, Gascon AR, et al. Size dependant oral inductions of immune response by administration of micro and nanospheres. Proceedings of the 27th International Symposium on Controlled Release of Bioactive Materials; 2000 Jul 7–13; Paris. Minneapolis (MN): Controlled Release Society, 2000
Guise V, Drouin JY, Benoit J, et al. Vidarabine-loaded nanoparticles: a physico-chemical study. Pharm Res 1990; 7: 736–41
Couvreur P, Vauthier C. Polyalkylcyanoacrylate nanoparticles as drug carrier: present state and perspectives. J Control Release 1991; 17: 187–98
Allemann E, Gurny R, Doelker E. Drug-loaded nanoparticles-preparation methods and drug targeting issues. Eur J Pharm Biopharm 1993; 39: 173–91
Alonso MJ. Nanoparticulate drug carrier technology. In: Cohen S, Bernstein H, editors. Microparticulate systems for the delivery of proteins and vaccines. New York: Marcel Dekker, 1996: 203–42
Couvreur P, Roland E, Speiser P. Biodegradable submicroscopic particles containing a biologically active substance and compositions containing them. US patent 4,329,332. 1982
El-Samaligy MS, Rohdewald P. Triamcinolone diacetate nanoparticles, a sustained release drug delivery system suitable for parenteral administration. Pharm Acta Helv 1982; 57: 201–4
Alonso MJ, Losa C, Calvo P, et al. Approaches to improve the association of amikacin sulfate to poly(alkylcyanoacrylate) nanoparticles. Int J Pharm 1991; 68: 69–76
Chavany C, Saison-Behmoaras T, Doan TL, et al. Adsorption of oligonucleotides onto polyisohexylcyanoacrylate nanoparticles protects them against nucleases and increase their cellular uptake. Pharm Res 1994; 11(9): 1370–8
Hillery AM, Toth I, Shaw AJ, et al. Co-polymerised peptide particles (CPP): I. Synthesis, characterisation and in vitro studies on a novel oral nanoparticulate delivery system. J Control Release 1996; 41: 271–81
Lindmann B, Stilbs P, Moseley M. Fourier transform NMR self-diffusion and microemulsion structure. J Colloid Interface Sci 1981; 83: 569–82
Kreilgaard M, Pedersen E, Jaroszewski JW. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release 2000; 69: 421–33
Pitaksuteepong T, Davies NM, Tucker IG, et al. Factors influencing the entrapment of hydrophilic compounds in nanocapsules prepared by interfacial polymerisation of water-in-oil microemulsions. Eur J Pharm Biopharm 2002; 53: 335–42
Pitaksuteepong T. Evaluation of nanocapsules for antigen delivery [thesis]. Dunedin: University of Otago, 2002
Grangier JL, Puygrenier M, Gautier JC, et al. Nanoparticles as carriers for growth hormone releasing factor. J Control Release 1991; 15: 3–13
Gibaud S, Rousseau C, Weingarten C, et al. Polyalkylcyanoacrylate nanoparticles as carriers for granulocyte-colony stimulating factor (G-CSF). J Control Release 1998; 52: 131–9
Wohlgemut M, Maechtle W, Mayer C. Improved preparation and physical studies of polycyanoacrylate nanocapsules. J Microencapsul 2000; 17: 437–48
Ilium L, Khan MA, Mak E, et al. Evaluation of carrier capacity and release characteristics for poly(butyl 2-cyanoacrylate) nanoparticles. Int J Pharm 1986; 30: 17–28
Fresta M, Puglisi G, Giammona G, et al. Perfloxacine mesilate- and ofloxacin-loaded polyethylcyanoacrylate nanoparticles: characterization of the colloidal drug carrier formulation. J Pharm Sci 1995; 84: 895–902
Boudad H, Legrand P, Lebas G, et al. Combined hydroxypropyl-beta-cyclodextrin and poly(alkylcyanoacrylate) nanoparticles intended for oral administration of saquinavir. Int J Pharm 2001; 218: 113–24
Monza da Silveira A, Ponchel G, Puisieux F, et al. Combined poly(isobutylcyanoacrylate) and cyclodextrins nanoparticles for enhancing the encapsulation of lipophilic drugs. Pharm Res 1998; 15: 1051–5
Mayer C, Hoffmann D, Wohlgemuth M. Structural analysis of nanocapsules by nuclear magnetic resonance. Int J Pharm 2002; 242: 37–46
Brasseur N, Brault D, Couvreur P. Adsorption of hematoporphyrin onto polyalkylcyanoacrylate nanoparticles: carrier capacity and drug release. Int J Pharm 1991; 70: 129–35
Peracchia MT, Vauthier C, Passirani C, et al. Complement consumption by poly (ethylene glycol) in different conformations chemically coupled to poly (isobutyl 2-cyanoacrylate) nanoparticles. Life Sci 1997; 61: 749–61
Bonduelle S, Foucher C, Leroux JC, et al. Association of cyclosporin in isohexylcyanoacrylate nanospheres and subsequent release in human plasma in vitro. J Microencapsul 1992; 9: 173–82
Ammoury N, Fessi H, Devissaguet JP, et al. Effect on cerebral blood flow of orally administered indomethacin-loaded poly(isobutylcyanoacrylate) and poly(DL-lactide) nanocapsules. J Pharm Pharmacol 1990; 42: 558–61
Zhang Q, Shen Z, Nagai T. Prolonged hypoglycemic effect of insulin-loaded polybutylcyanoacrylate nanoparticles after pulmonary administration to normal rats. Int J Pharm 2001; 218: 75–80
Kreuter J, Alyautdin RN, Kharkevich DA, et al. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res 1995; 674: 171–4
Kreuter J. Nanoparticulate delivery systems for the brain delivery of drugs. Adv Drug Deliv Rev 2001; 47: 65–81
Alyautdin RN, Petrov VE, Langer K, et al. Delivery of loperamide across the blood-brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm Res 1997; 14: 325–8
Olivier J-C, Fenart L, Chauvet R, et al. Indirect evidence that drug brain targeting using polysorbate 80-coated polyisobutylcyanoacrylate nanoparticles is related to toxicity. Pharm Res 1999; 16: 1836–42
Kattan J, Droz JP, Couvreur P, et al. Phase I clinical trial and pharmacokinetic evaluation of doxorubicin carried by polyisohexylcyanoacrylate nanoparticles. Invest New Drugs 1992; 10: 191–9
Acknowledgment
The authors would like to thank the School of Pharmacy, University of Otago, for supplying a scholarship to Karen Krauel, the University of Otago for supplying a scholarship to Tasana Pitaksuteepong and the New Zealand Pharmacy Education and Research Foundation and Otago Research Grants for funding aspects of the work described. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krauel, K., Pitaksuteepong, T., Davies, N.M. et al. Entrapment of bioactive molecules in poly (alkylcyanoacrylate) nanoparticles. Am J Drug Deliv 2, 251–259 (2004). https://doi.org/10.2165/00137696-200402040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00137696-200402040-00005