Abstract
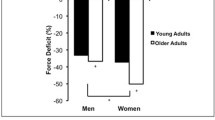
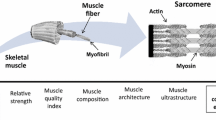
Skeletal muscle comprises the largest organ system in the human body and is essential for force generation and movement. Skeletal muscle is subjected to considerable stresses during everyday use. However, muscle has the unique ability to adapt and remodel to provide protection against such stresses. This adaptation occurs at the structural through to the cellular level, which includes changes in transcription of a range of protective proteins. Failure in such processes can be catastrophic. This failure in adaptation is particularly notable in older individuals. Our skeletal muscles become smaller and weaker as we age. This loss of muscle bulk results in a reduced capacity to generate force and results in a loss of the ability to undertake everyday tasks. This article describes the normal adaptive responses of muscle in younger individuals to the stress of various forms of exercise and the implications of a failure of these adaptive responses in the elderly.
Similar content being viewed by others
References
Newham DJ, Mills KR, Quigley BM, et al. Pain and fatigue after concentric and eccentric muscle contractions. Clin Sci (Lond) 1983; 64 (1): 55–62
Faulkner JA. Terminology for contractions of muscles during shortening, while isometric, and during lengthening. J Appl Physiol 2003; 95 (2): 455–9
Clarkson PM, Nosako K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 1992; 24 (5): 512–50
McCully KK, Faulkner JA. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol 1985; 59 (1): 119–26
McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol 1986; 61 (1): 293–9
Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol 2001; 537 (2): 335–45
Faulkner JA, Brooks SV, Zerba E. Skeletal muscle weakness and fatigue in old age: underlying mechanisms. Annu Rev Gerontol Geriatr 1990; 10: 147–66
Jones DA, Round JM. Human muscle damage induced by eccentric exercise or reperfusion injury: a common mechanism. In: Salmons S, editor. Muscle damage. Oxford: Oxford University Press, 1997: 64–75
Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol 1995; 488 (Pt 2): 459–69
Faulkner JA, Brooks SV. Muscle damage induced by contraction: an in situ single skeletal muscle model. Salmons S, editor. Oxford: Oxford University Press, 1997
McArdle A, Dillmann WH, Mestril R, et al. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 2004; 18 (2): 355–7
Eston RG, Mickleborough J, Baltzopoulos V. Eccentric activation and muscle damage: biomechanical and physiological considerations during downhill running. Br J Sports Med 1995; 29 (2): 89–94
McArdle A, Jackson MJ. Intracellular mechanisms involved in skeletal muscle damage. Salmons S, editor. Oxford: Oxford University Press, 1997: 90–106
Maglara A, Jackson MJ, McArdle A. Programmed cell death in skeletal muscle [abstract]. Biochem Soc Trans 1998; 26 (3): S259
McArdle A, Maglara A, Appleton P, et al. Apoptosis in multinucleated skeletal muscle myotubes. Lab Invest 1999; 79 (9): 1069–76
Jackson MJ, O’Farrell S. Free radicals and muscle damage. Br Med Bull 1993; 49 (3): 630–41
Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide: general properties and effect of hyperbaric oxygen. Biochem J 1973; 134 (3): 707–16
Jackson MJ, Edwards RHT, Symons MCR. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta 1985; 847: 184–90
McArdle A, Pattwell D, Vasilaki A, et al. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 2001; 280 (3): C621–7
O’Neill CA, Stebbins CL, Bonigut S, et al. Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol 1996; 81 (3): 1197–206
Reid MB, Shoji T, Moody MR, et al. Reactive oxygen in skeletal muscle II: extracellular release of free radicals. J Appl Physiol 1992; 73 (5): 1805–9
Close GL, Ashton T, Cable T, et al. Eccentric exercise, isokinetic muscle torque and delayed onset muscle soreness: the role of reactive oxygen species. Eur J Appl Physiol 2004; 91 (5–6): 615–21
Sen CK. Antioxidants in exercise nutrition. Sports Med 2001; 31 (13): 891–908
Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr 1991; 53 (4 Suppl.): 1050S-5S
Sumida S, Tanaka K, Kitao H, et al. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int J Biochem 1989; 21 (8): 835–8
McBride JM, Kraemer WJ, Triplett-McBride T, et al. Effect of resistance exercise on free radical production. Med Sci Sports Exerc 1998; 30 (1): 67–72
Jakeman P, Maxwell S. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur J Appl Physiol Occup Physiol 1993; 67 (5): 426–30
Kaminski M, Boal R. An effect of ascorbic acid on delayed-onset-muscle-soreness. Pain 1992; 50 (3): 317–21
Staton WM. The influence of ascorbic acid supplementation in minimizing post-exercise muscle soreness in young men. Res Q 1952; 23: 356–60
Thompson D, Williams C, Kingsley M, et al. Muscle soreness and damage parameters after prolonged intermittent shuttle-running following acute vitamin C supplementation. Int J Sports Med 2001; 22 (1): 68–75
Close GL, Ashton T, Cable NT, et al. Prolonged ascorbic acid supplementation attenuates post-exercise lipid peroxidation but has no effect on delayed onset muscle soreness following downhill running in man [abstract]. J Physiol 2004; 555P: PC95
McArdle A, van der Meulen JH, Catapano M, et al. Free radical activity following contraction-induced injury to the extensor digitorum longus muscles of rats. Free Radic Biol Med 1999; 26 (9/10): 1085–91
van der Meulen JH, McArdle A, Jackson MJ, et al. Contraction-induced injury to the extensor digitorum longus muscles of rats: the role of vitamin E. J Appl Physiol 1997; 83 (3): 817–23
Cannon JG, Orencole SF, Fielding RA, et al. Acute phase response in exercise: interaction of age and vitamin E on neutrophils and muscle enzyme release. Am J Physiol 1990; 259 (6 Pt 2): R1214–9
Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol 1994; 77 (6): 2519–21
Ji LL. Antioxidant enzyme response to exercise and aging. Med Sci Sports Exerc 1993; 25 (2): 225–31
Higuchi M, Cartier LJ, Chen M, et al. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol 1985; 40 (3): 281–6
Jenkins RR, Friedland R, Howald H. The relationship of oxygen uptake to superoxide dismutase and catalase activity in human skeletal muscle. Int J Sports Med 1984; 5 (1): 11–4
Khassaf M, Child RB, McArdle A, et al. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J Appl Physiol 2001; 90 (3): 1031–5
Radak Z. Free radicals in exercise and ageing. Leeds: Human Kinetics, 2000: ix
Robertson JD, Maughan RJ, Duthie GG, et al. Increased blood antioxidant systems of runners in response to training load. Clin Sci (Lond) 1991; 80 (6): 611–8
Vertuani S, Angusti A, Manfredini S. The antioxidants and pro-antioxidants network: an overview. Curr Pharm Des 2004; 10 (14): 1677–94
Powers SK, DeRuisseau KC, Quindry J, et al. Dietary antioxidants and exercise. J Sports Sci 2004; 22 (1): 81–94
Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol 1995; 79 (3): 675–86
McArdle A, Jackson MJ. Stress proteins and exercise-induced muscle damage. Boca Raton (FL): CRC Press, 2002: 137–50
Locke M, Noble EG. Exercise and stress response: the role of stress proteins. Boca Raton (FL): CRC Press, 2002
Hernando R, Manso R. Muscle fibre stress in response to exercise: synthesis, accumulation and isoform transitions of 70-kDa heat-shock proteins. Eur J Biochem 1997; 243 (1–2): 460–7
Kelly DA, Tiidus PM, Houston ME, et al. Effect of vitamin E deprivation and exercise training on induction of HSP70. J Appl Physiol 1996; 81 (6): 2379–85
Salo DC, Donovan CM, Davies KJ. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med 1991; 11 (3): 239–46
Samelman TR. Heat shock protein expression is increased in cardiac and skeletal muscles of Fischer 344 rats after endurance training. Exp Physiol 2000; 85 (1): 92–102
Naito H, Powers SK, Demirel HA, et al. Exercise training increases heat shock protein in skeletal muscles of old rats. Med Sci Sports Exerc 2001; 33 (5): 729–34
Freeman ML, Borrelli MJ, Meredith MJ, et al. On the path to the heat shock response: destabilization and formation of partially folded protein intermediates, a consequence of protein thiol modification. Free Radic Biol Med 1999; 26 (5–6): 737–45
Marber MS, Mestril R, Chi SH, et al. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest 1995; 95 (4): 1446–56
Radford NB, Fina M, Benjamin IJ, et al. Cardioprotective effects of 70-kDa heat shock protein in transgenic mice. Proc Natl Acad Sci U S A 1996; 93 (6): 2339–42
Rajdev S, Hara K, Kokubo Y, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol 2000; 47 (6): 782–91
Belcastro AN, Shewchuk LD, Raj DA. Exercise-induced muscle injury: a calpain hypothesis. Mol Cell Biochem 1998; 179 (1–2): 135–45
Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 1995; 27 (7): 1022–32
Merly F, Lescaudron L, Rouaud T, et al. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 1999; 22 (6): 724–32
Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 1961; 9: 493–5
Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 1982; 202 (3): 329–37
Wokke JH, Van den Oord CJ, Leppink GJ, et al. Perisynaptic satellite cells in human external intercostal muscle: a quantitative and qualitative study. Anat Rec 1989; 223 (2): 174–80
Schmalbruch H, Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec 1977; 189 (2): 169–75
Shultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 1985; 8: 217–22
Shultz E, McCormick KM. Skeletal muscle satellite cells. Rev Physiol Biochem 1994; 123: 213–57
Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 2001; 91 (2): 534–51
Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol 2003; 35: 1151–6
Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 1988; 404: 71–82
Goldspink G. Cellular and molecular aspects of adaptation in skeletal muscle. Komi PV, editor. London: Blackwell Science, 1994: 211–29
McBride TA, Gorin FA, Carlsen RC. Prolonged recovery and reduced adaptation in aged rat muscle following eccentric exercise. Mech Ageing Dev 1995; 83 (3): 185–200
Schwane JA, Johnson SR, Vandernakker CB, et al. Delayed onset muscle soreness and plasma CPK and LDH activities after downhill running. Med Sci Sports Exerc 1983; 15 (1): 51–6
Schwane JA, Williams JS, Sloan JH. Effects of training on delayed muscle soreness and serum creatine kinase activity after running. Med Sci Sports Exerc 1987; 19 (6): 584–90
Pierrynowski MR, Tudus PM, Plyley MJ. Effects of downhill or uphill training prior to a downhill run. Eur J Appl Physiol 1987; 56: 668–72
Brown SJ, Child RB, Day SH, et al. Exercise induced muscle damage and adaptation following repeated bouts of eccentric muscle contraction. J Sports Sci 1997; 15: 215–22
Whitehead NP, Allen TJ, Morgan DL, et al. Damage to human muscle from eccentric exercise after training with concentric exercise. J Physiol 1998; 512 (Pt 2): 615–20
McHugh MP, Pasiakos S. The role of exercising muscle length in the protective adaptation to a single bout of eccentric exercise. Eur J Appl Physiol. Epub 2004 Aug 27
Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 2001; 281 (1): R155–61
McHugh MP, Connolly DAJ, Eston RG, et al. Exercise induced muscle damage and potential mechanisms for the repeated bout effect. Sports Med 1999; 27 (3): 157–70
Lapointe BM, Fremont P, Cote CH. Adaptation to lengthening contractions is independent of voluntary muscle recruitment but relies on inflammation. Am J Physiol Regul Integr Comp Physiol 2002; 282 (1): R323–9
Devor ST, Faulkner JA. Regeneration of new fibers in muscles of old rats reduces contraction-induced injury. J Appl Physiol 1999; 87 (2): 750–6
Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 1995; 5 (3): 129–42
Sato T, Akatsuka H, Kito K, et al. Age changes in size and number of muscle fibers in human minor pectoral muscle. Mech Ageing Dev 1984; 28 (1): 99–109
Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles: fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci 1986; 72 (2–3): 211–22
Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 1988; 84 (2–3): 275–94
Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand 1978; 104 (2): 129–36
Larsson L. Morphological and functional characteristics of the ageing skeletal muscle in man: a cross-sectional study. Acta Physiol Scand Suppl 1978; 457: 1–36
Stanley SN, Taylor NA. Isokinematic muscle mechanics in four groups of women of increasing age. Eur J Appl Physiol Occup Physiol 1993; 66 (2): 178–84
Faulkner JA, Brooks SV, Zerba E. Skeletal muscle weakness, fatigue, and injury: inevitable concomitants of aging? In: Ghesquire J, Tolleneer J, editors. Leuven: Liber Amicorum – Hermes XXI, 1990: 269–80
Faulkner JA, White TP. Changes that occur and do not occur in the structure and function of skeletal muscle with aging. J Gerontol 1988; 43 (1): B3–4
Evans WJ. Exercise training guidelines for the elderly. Med Sci Sports Exerc 1999; 31 (1): 12–7
Ploutz-Snyder LL, Giamis EL, Formikell M, et al. Resistance training reduces susceptibility to eccentric exercise-induced muscle dysfunction in older women. J Gerontol A Biol Sci Med Sci 2001; 56 (9): B384–90
Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med 1989; 7 (4): 207–34
Nosako K, Clarkson PM. Changes in indicators of inflammation after eccentric exercise of the elbow flexors. Med Sci Sports Exerc 1996; 28 (8): 953–61
Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle Nerve 1983; 6 (8): 574–80
Renault V, Thornell LE, Butler-Browne G, et al. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol 2002; 37 (10–11): 1229–36
Renault V, Piron-Hamelin G, Forestier C, et al. Skeletal muscle regeneration and the mitotic clock. Exp Gerontol 2000; 35 (6–7): 711–9
Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol 1990; 258 (3 Pt 1): C429–35
Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol 1989; 256 (6 Pt 1): C1262–6
Liu AYC, Lee YK, Manola LD, et al. Attenuated heat shock transcriptional response in ageing: molecular mechanism and implication in the biology of ageing. In: Feige U, Morimoto RI, Yahara I, et al., editors. Berlin: Birkhauser, 1996: 393–408
Rao DV, Watson K, Jones GL. Age-related attenuation in the expression of the major heat shock proteins in human peripheral lymphocytes. Mech Ageing Dev 1999; 107 (1): 105–18
Vasilaki A, Jackson MJ, McArdle A. Attenuated HSP70 response in skeletal muscle of aged rats following contractile activity. Muscle Nerve 2002; 25 (6): 902–5
Poon HF, Calabrese V, Scapagnini G, et al. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci 2004; 59 (5): 478–93
Zhang J, Dai J, Lu Y, et al. In vivo visualization of aging-associated gene transcription: evidence for free radical theory of aging. Exp Gerontol 2004; 39 (2): 239–47
Meissner C, Mohamed SA, von Wurmb N, et al. The mitochondrial genome and aging. Z Gerontol Geriatr 2001; 34 (6): 447–51
de Grey AD. The reductive hotspot hypothesis: an update. Arch Biochem Biophys 2000; 373 (1): 295–301
de Grey AD. The reductive hotspot hypothesis of mammalian aging: membrane metabolism magnifies mutant mitochondrial mischief. Eur J Biochem 2002; 269 (8): 2003–9
Sacco P, Jones DA. The protective effect of damaging eccentric exercise against repeated bouts of exercise in the mouse tibialis anterior muscle. Exp Physiol 1992; 77 (5): 757–60
Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J Gerontol A Biol Sci Med Sci 2001; 56 (4): B163–71
Gosselin LE. Attenuation of force deficit after lengthening contractions in soleus muscle from trained rats. J Appl Physiol 2000; 88 (4): 1254–8
Fielding RA, LeBrasseur NK, Cuoco A, et al. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc 2002; 50 (4): 655–62
Schulte JN, Yarasheski KE. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int J Sport Nutr Exerc Metab 2001; 11 Suppl.: S111–8
Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci 2002; 57 (4): B138–43
Lambert CP, Sullivan DH, Freeling SA, et al. Effects of testosterone replacement and/or resistance exercise on the composition of megestrol acetate stimulated weight gain in elderly men: a randomized controlled trial. J Clin Endocrinol Metab 2002; 87 (5): 2100–6
Newton RU, Hakkinen K, Hakkinen A, et al. Mixed-methods resistance training increases power and strength of young and older men. Med Sci Sports Exerc 2002; 34 (8): 1367–75
Ferri A, Scaglioni G, Pousson M, et al. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand 2003; 177 (1): 69–78
Kalapotharakos VI, Michalopoulou M, Godolias G, et al. The effects of high- and moderate-resistance training on muscle function in the elderly. J Aging Phys Act 2004; 12 (2): 131–43
Hakkinen K, Kraemer WJ, Pakarinen A, et al. Effects of heavy resistance/power training on maximal strength, muscle morphology, and hormonal response patterns in 60–75-year-old men and women. Can J Appl Physiol 2002; 27 (3): 213–31
Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci 2003; 58 (10): M918–22
Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med 2004; 34 (5): 329–48
Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol 2003; 95 (4): 1717–27
Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 2004; 91 (4): 450–72
Capodaglio P, Facioli M, Burroni E, et al. Effectiveness of a home-based strengthening program for elderly males in Italy: a preliminary study. Aging Clin Exp Res 2002; 14 (1): 28–34
Smith K, Winegard K, Hicks AL, et al. Two years of resistance training in older men and women: the effects of three years of detraining on the retention of dynamic strength. Can J Appl Physiol 2003; 28 (3): 462–74
Acknowledgements
The authors would like to thank the Biotechnology and Biological Sciences Research Council, Research Into Ageing, and the Wellcome Trust for providing funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Close, G.L., Kayani, A., Vasilaki, A. et al. Skeletal Muscle Damage with Exercise and Aging. Sports Med 35, 413–427 (2005). https://doi.org/10.2165/00007256-200535050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-200535050-00004